Blockade of CTLA-4 on both effector and regulatory T cell compartments contributes to the antitumor activity of anti-CTLA-4 antibodies
- PMID: 19581407
- PMCID: PMC2722174
- DOI: 10.1084/jem.20082492
Blockade of CTLA-4 on both effector and regulatory T cell compartments contributes to the antitumor activity of anti-CTLA-4 antibodies
Abstract
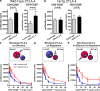
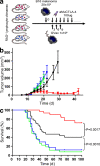
Cytotoxic T lymphocyte-associated antigen 4 (CTLA-4) is a critical negative regulator of immune responses. Uniquely among known inhibitory receptors, its genetic ablation results in a fulminating and fatal lymphoproliferative disorder. This central regulatory role led to the development of antibodies designed to block CTLA-4 activity in vivo, aiming to enhance immune responses against cancer. Despite their preclinical efficacy and promising clinical activity against late stage metastatic melanoma, the critical cellular targets for their activity remains unclear. In particular, debate has focused on whether the effector T cell (T(eff)) or regulatory T cell (T reg cell) compartment is the primary target of antibody-mediated blockade. We developed a mouse expressing human instead of mouse CTLA-4, allowing us to evaluate the independent contributions of CTLA-4 blockade of each T cell compartment during cancer immunotherapy in an in vivo model of mouse melanoma. The data show that although blockade on effector cells significantly improves tumor protection, unicompartmental blockade on regulatory cells completely fails to enhance antitumor responses. However, concomitant blockade of both compartments leads to a synergistic effect and maximal antitumor activity. We conclude that the combination of direct enhancement of T(eff) cell function and concomitant inhibition of T reg cell activity through blockade of CTLA-4 on both cell types is essential for mediating the full therapeutic effects of anti-CTLA-4 antibodies during cancer immunotherapy.
Figures


References
-
- Bachmann M.F., Kohler G., Ecabert B., Mak T.W., Kopf M. 1999. Cutting edge: lymphoproliferative disease in the absence of CTLA-4 is not T cell autonomous.J. Immunol. 163:1128–1131 - PubMed
-
- Bachmann M.F., Gallimore A., Jones E., Ecabert B., Acha-Orbea H., Kopf M. 2001. Normal pathogen-specific immune responses mounted by CTLA-4-deficient T cells: a paradigm reconsidered.Eur. J. Immunol. 31:450–458 - PubMed
-
- Bodor J., Fehervari Z., Diamond B., Sakaguchi S. 2007. ICER/CREM-mediated transcriptional attenuation of IL-2 and its role in suppression by regulatory T cells.Eur. J. Immunol. 37:884–895 - PubMed
-
- Carreno B.M., Bennett F., Chau T.A., Ling V., Luxenberg D., Jussif J., Baroja M.L., Madrenas J. 2000. CTLA-4 (CD152) can inhibit T cell activation by two different mechanisms depending on its level of cell surface expression.J. Immunol. 165:1352–1356 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

