Mitochondrial networking protects beta-cells from nutrient-induced apoptosis
- PMID: 19581419
- PMCID: PMC2750232
- DOI: 10.2337/db07-1781
Mitochondrial networking protects beta-cells from nutrient-induced apoptosis
Abstract
Objective: Previous studies have reported that beta-cell mitochondria exist as discrete organelles that exhibit heterogeneous bioenergetic capacity. To date, networking activity, and its role in mediating beta-cell mitochondrial morphology and function, remains unclear. In this article, we investigate beta-cell mitochondrial fusion and fission in detail and report alterations in response to various combinations of nutrients.
Research design and methods: Using matrix-targeted photoactivatable green fluorescent protein, mitochondria were tagged and tracked in beta-cells within intact islets, as isolated cells and as cell lines, revealing frequent fusion and fission events. Manipulations of key mitochondrial dynamics proteins OPA1, DRP1, and Fis1 were tested for their role in beta-cell mitochondrial morphology. The combined effects of free fatty acid and glucose on beta-cell survival, function, and mitochondrial morphology were explored with relation to alterations in fusion and fission capacity.
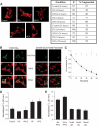
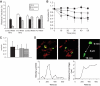
Results: beta-Cell mitochondria are constantly involved in fusion and fission activity that underlies the overall morphology of the organelle. We find that networking activity among mitochondria is capable of distributing a localized green fluorescent protein signal throughout an isolated beta-cell, a beta-cell within an islet, and an INS1 cell. Under noxious conditions, we find that beta-cell mitochondria become fragmented and lose their ability to undergo fusion. Interestingly, manipulations that shift the dynamic balance to favor fusion are able to prevent mitochondrial fragmentation, maintain mitochondrial dynamics, and prevent apoptosis.
Conclusions: These data suggest that alterations in mitochondrial fusion and fission play a critical role in nutrient-induced beta-cell apoptosis and may be involved in the pathophysiology of type 2 diabetes.
Figures
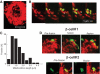



 and ▤, respectively. D: Confocal images demonstrating mitochondrial movement analysis. Images at time 0 and 10 min are portrayed. The last image zooms to one mitochondrion and the movement over 10 min is depicted, in this case 4 μm over 10 min. The velocity over time is plotted and indicates a peak around 250 s. When the distance from the origin is plotted over time, it is evident that mitochondria do not move at a constant speed. (A high-quality digital representation of this figure is available in the online issue.)
and ▤, respectively. D: Confocal images demonstrating mitochondrial movement analysis. Images at time 0 and 10 min are portrayed. The last image zooms to one mitochondrion and the movement over 10 min is depicted, in this case 4 μm over 10 min. The velocity over time is plotted and indicates a peak around 250 s. When the distance from the origin is plotted over time, it is evident that mitochondria do not move at a constant speed. (A high-quality digital representation of this figure is available in the online issue.)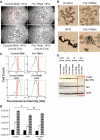
Comment in
- Diabetes. 58:2185.
References
-
- Skulachev VP: Mitochondrial filaments and clusters as intracellular power-transmitting cables. Trends Biochem Sci 2001; 26: 23– 29 - PubMed
-
- Frank S: Dysregulation of mitochondrial fusion and fission: an emerging concept in neurodegeneration. Acta Neuropathol (Berl) 2006; 111: 93– 100 - PubMed
-
- Nakada K, Inoue K, Ono T, Isobe K, Ogura A, Goto YI, Nonaka I, Hayashi JI: Inter-mitochondrial complementation: mitochondria-specific system preventing mice from expression of disease phenotypes by mutant mtDNA. Nat Med 2001; 7: 934– 940 - PubMed
-
- Chen H, Chomyn A, Chan DC: Disruption of fusion results in mitochondrial heterogeneity and dysfunction. J Biol Chem 2005; 280: 26185– 26192 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

