Phase II trial of conformal radiation therapy for pediatric low-grade glioma
- PMID: 19581536
- PMCID: PMC3525947
- DOI: 10.1200/JCO.2008.20.9494
Phase II trial of conformal radiation therapy for pediatric low-grade glioma
Abstract
Purpose: The use of radiotherapy in pediatric low-grade glioma (LGG) is controversial, especially for young patients. We conducted a phase II trial of conformal radiation therapy (CRT) to estimate disease control by using a 10-mm clinical target volume (CTV) margin.
Materials and methods: Between August 1997 and August 2006, 78 pediatric patients with LGG and a median age of 8.9 years (range, 2.2 to 19.8 years) received 54 Gy CRT by using a 10-mm CTV and by targeting with systematic magnetic resonance imaging (MRI) registration. Tumor locations were diencephalon (n = 58), cerebral hemisphere (n = 3), and cerebellum (n = 17). Sixty-seven patients had documented or presumed WHO grade 1 tumors, 25 patients had prior chemotherapy, and 13 patients had neurofibromatosis type 1.
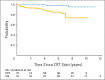
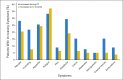
Results: During a median follow-up of 89 months, 13 patients experienced disease progression. One patient experienced marginal treatment failure, eight experienced local failures, and four experienced metastatic failure. The mean and standard error 5- and 10-year event-free (87.4% +/- 4.4% and 74.3% +/- 15.4%, respectively) and overall (98.5% +/- 1.6% and 95.9% +/- 5.8%, respectively) survival rates were determined. The mean and standard error cumulative incidences of local failure at 5 and 10 years were 8.7% +/- 3.5% and 16.4% +/- 5.4%, respectively. The mean and standard error cumulative incidence of vasculopathy was 4.79% +/- 2.73% at 6 years, and it was higher for those younger than 5 years of age (P = .0105) at the time of CRT.
Conclusion: This large, prospective series of irradiated children with LGG demonstrates that CRT with a 10-mm CTV does not compromise disease control. The results suggest that CRT should be delayed in young patients to reduce the risk of vasculopathy.
Conflict of interest statement
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
Figures


References
-
- Gnekow A, De Salvo GL, Thieme B, et al. SIOP-LGG 2004: Comprehensive treatment strategy for low-grade glioma in children and adolescents, including a randomized chemotherapy trial and a radiotherapy trial. Neuro Oncol. 2008;10:452–453. abstr LGG 21.
-
- National Cancer Institute, National Institutes of Health. Phase II Study of Reduced-Field Conformal Radiotherapy in Young Patients With Low-Grade Gliomas. http://www.cancer.gov/clinicaltrials/COG-ACNS0221.
-
- Brauner R, Malandry F, Rappaport R, et al. Growth and endocrine disorders in optic glioma. Eur J Pediatr. 1990;149:825–828. - PubMed
-
- Janss AJ, Grundy R, Cnaan A, et al. Optic pathway and hypothalamic/chiasmatic gliomas in children younger than age 5 years with a 6-year follow-up. Cancer. 1995;75:1051–1059. - PubMed
-
- Chadderton RD, West CG, Schuller S, et al. Radiotherapy in the treatment of low-grade astrocytomas: II. The physical and cognitive sequelae. Childs Nerv Syst. 1995;11:443–448. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Research Materials

