Characterization and culture of fetal rhesus monkey renal cortical cells
- PMID: 19581826
- PMCID: PMC3175418
- DOI: 10.1203/PDR.0b013e3181b45565
Characterization and culture of fetal rhesus monkey renal cortical cells
Abstract
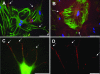
The renal glomerulus is composed of endothelial and mesangial cells with podocytes contributing to glomerular filtration. Podocyte damage is associated with renal disorders, thus there is interest in these cells for regenerative medicine. These studies investigated the use of extracellular matrix (ECM) to grow third trimester fetal monkey renal cortical cells and to assess mature podocytes in culture. Immunohistochemistry provided a profile of podocyte differentiation with metanephric mesenchyme and developing podocytes nestin positive and synaptopodin negative, whereas mature podocytes were positive for both markers. Primary cell cultures devoid of mature podocytes were established on plastic and renal ECM. A cell population (nestin+/synatopodin-) cultured on renal ECM showed greater proliferative potential compared with plastic with limited podocytes developing in culture over time. Further investigation of individual components of ECM (laminin, fibronectin, collagen I, or collagen IV) indicated that collagen I supported the greatest proliferation similar to renal ECM, whereas a greater number of mature podocytes (nestin+/synaptopodin+) were observed on fibronectin. These results suggest that (1) culture of fetal monkey podocytes can be accomplished, (2) renal ECM and collagen I can support renal cortical cells in vitro, which may recapitulate the developing kidney in vivo, and (3) fibronectin can support podocyte differentiation in vitro.
Figures





References
-
- Quaggin SE, Kreidberg JA. Development of the renal glomerulus: good neighbors and good fences. Development. 2008;135:609–620. - PubMed
-
- Saxen L. Organogenesis of the kidney. Cambridge University Press; Cambridge: 1987. pp. 135–142.
-
- Miner JH. Renal basement membrane components. Kidney Int. 1999;56:2016–2024. - PubMed
-
- Haller H, de Groot K, Bahlmann F, Elger M, Fliser D. Stem cells and progenitor cells in renal disease. Kidney Int. 2005;68:1932–1936. - PubMed
-
- Bertelli E, Regoli M, Fonzi L, Occhini R, Mannucci S, Ermini L, Toti P. Nestin expression in adult and developing human kidney. J Histochem Cytochem. 2007;55:411–421. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

