Towards single-molecule nanomechanical mass spectrometry
- PMID: 19581898
- PMCID: PMC3846395
- DOI: 10.1038/nnano.2009.152
Towards single-molecule nanomechanical mass spectrometry
Abstract
Mass spectrometry provides rapid and quantitative identification of protein species with relatively low sample consumption. The trend towards biological analysis at increasingly smaller scales, ultimately down to the volume of an individual cell, continues, and mass spectrometry with a sensitivity of a few to single molecules will be necessary. Nanoelectromechanical systems provide unparalleled mass sensitivity, which is now sufficient for the detection of individual molecular species in real time. Here, we report the first demonstration of mass spectrometry based on single biological molecule detection with a nanoelectromechanical system. In our nanoelectromechanical-mass spectrometry system, nanoparticles and protein species are introduced by electrospray injection from the fluid phase in ambient conditions into vacuum, and are subsequently delivered to the nanoelectromechanical system detector by hexapole ion optics. Precipitous frequency shifts, proportional to the mass, are recorded in real time as analytes adsorb, one by one, onto a phase-locked, ultrahigh-frequency nanoelectromechanical resonator. These first nanoelectromechanical system-mass spectrometry spectra, obtained with modest mass sensitivity from only several hundred mass adsorption events, presage the future capabilities of this approach. We also outline the substantial improvements that are feasible in the near term, some of which are unique to nanoelectromechanical system based-mass spectrometry.
Figures
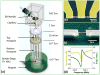

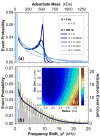
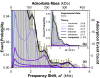
Comment in
-
Nanoelectromechanical systems: Mass spec goes nanomechanical.Nat Nanotechnol. 2009 Jul;4(7):404-5. doi: 10.1038/nnano.2009.169. Nat Nanotechnol. 2009. PMID: 19581886 No abstract available.
References
-
- Roukes ML. Nanoelectromechanical systems face the future. Phys World. 2001 Feb;14:25–31.
-
- Naik A, et al. Cooling a nanomechanical resonator with quantum back-action. Nature. 2006;443:193–196. - PubMed
-
- Burg TP, Manalis SR. Suspended microchannel resonators for biomolecular detection. Appl Phys Lett. 2003;83:2698–2700.
-
- LaHaye MD, Buu O, Camarota B, Schwab KC. Approaching the quantum limit of a nanomechanical resonator. Science. 2004;304:74–77. - PubMed
-
- Thompson JD, et al. Strong dispersive coupling of a high-finesse cavity to a micromechanical membrane. Nature. 2008;452:72–75. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

