Repression of flowering by the miR172 target SMZ
- PMID: 19582143
- PMCID: PMC2701598
- DOI: 10.1371/journal.pbio.1000148
Repression of flowering by the miR172 target SMZ
Abstract
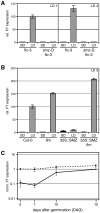
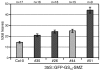
A small mobile protein, encoded by the FLOWERING LOCUS T (FT) locus, plays a central role in the control of flowering. FT is regulated positively by CONSTANS (CO), the output of the photoperiod pathway, and negatively by FLC, which integrates the effects of prolonged cold exposure. Here, we reveal the mechanisms of regulation by the microRNA miR172 target SCHLAFMUTZE (SMZ), a potent repressor of flowering. Whole-genome mapping of SMZ binding sites demonstrates not only direct regulation of FT, but also of many other flowering time regulators acting both upstream and downstream of FT, indicating an important role of miR172 and its targets in fine tuning the flowering response. A role for the miR172/SMZ module as a rheostat in flowering time is further supported by SMZ binding to several other genes encoding miR172 targets. Finally, we show that the action of SMZ is completely dependent on another floral repressor, FLM, providing the first direct connection between two important classes of flowering time regulators, AP2- and MADS-domain proteins.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures







References
-
- Putterill J, Laurie R, Macknight R. It's time to flower: the genetic control of flowering time. Bioessays. 2004;26:363–373. - PubMed
-
- Garner WW, Allard HA. Photoperiodism, the response of the plant to relative length of day and night. Science. 1922;55:582–583. - PubMed
-
- Imaizumi T, Kay SA. Photoperiodic control of flowering: not only by coincidence. Trends Plant Sci. 2006;11:550–558. - PubMed
-
- Sachs J. Wirkung des Lichtes auf die Blütenbildung unter Vermittlung der Laubblätter. Bot Ztg. 1865;23:117–121; 125–131; 133–139.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

