Polyclonal B cell differentiation and loss of gastrointestinal tract germinal centers in the earliest stages of HIV-1 infection
- PMID: 19582166
- PMCID: PMC2702159
- DOI: 10.1371/journal.pmed.1000107
Polyclonal B cell differentiation and loss of gastrointestinal tract germinal centers in the earliest stages of HIV-1 infection
Abstract
Background: The antibody response to HIV-1 does not appear in the plasma until approximately 2-5 weeks after transmission, and neutralizing antibodies to autologous HIV-1 generally do not become detectable until 12 weeks or more after transmission. Moreover, levels of HIV-1-specific antibodies decline on antiretroviral treatment. The mechanisms of this delay in the appearance of anti-HIV-1 antibodies and of their subsequent rapid decline are not known. While the effect of HIV-1 on depletion of gut CD4(+) T cells in acute HIV-1 infection is well described, we studied blood and tissue B cells soon after infection to determine the effect of early HIV-1 on these cells.
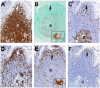
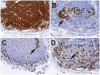
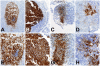
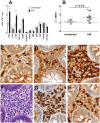
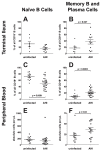
Methods and findings: In human participants, we analyzed B cells in blood as early as 17 days after HIV-1 infection, and in terminal ileum inductive and effector microenvironments beginning at 47 days after infection. We found that HIV-1 infection rapidly induced polyclonal activation and terminal differentiation of B cells in blood and in gut-associated lymphoid tissue (GALT) B cells. The specificities of antibodies produced by GALT memory B cells in acute HIV-1 infection (AHI) included not only HIV-1-specific antibodies, but also influenza-specific and autoreactive antibodies, indicating very early onset of HIV-1-induced polyclonal B cell activation. Follicular damage or germinal center loss in terminal ileum Peyer's patches was seen with 88% of follicles exhibiting B or T cell apoptosis and follicular lysis.
Conclusions: Early induction of polyclonal B cell differentiation, coupled with follicular damage and germinal center loss soon after HIV-1 infection, may explain both the high rate of decline in HIV-1-induced antibody responses and the delay in plasma antibody responses to HIV-1. Please see later in the article for Editors' Summary.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures




References
-
- Guadalupe M, Reay E, Sankaran S, Prindiville T, Flamm J, et al. Severe CD4+ T-cell depletion in gut lymphoid tissue during primary human immunodeficiency virus type 1 infection and substantial delay in restoration following highly active antiretroviral therapy. J Virol. 2003;77:11708–11717. - PMC - PubMed
-
- Veazey RS, Marx PA, Lackner AA. The mucosal immune system: primary target for HIV infection and AIDS. Trends Immunol. 2001;22:626–633. - PubMed
-
- Pope M, Haase AT. Transmission, acute HIV-1 infection and the quest for strategies to prevent infection. Nat Med. 2003;9:847–852. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

