Effect of a vitamin D(3) derivative (B3CD) with postulated anti-cancer activity in an ovarian cancer animal model
- PMID: 19582372
- PMCID: PMC2904825
- DOI: 10.1007/s10637-009-9284-y
Effect of a vitamin D(3) derivative (B3CD) with postulated anti-cancer activity in an ovarian cancer animal model
Abstract
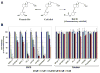
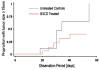
The objective of the present study was to test the hypothesis that Calcidiol derivative B3CD qualifies as a potential anti-cancer drug in vivo employing an ovarian cancer xenograft model in mice. In addition, the selectivity of B3CD on viability and proliferation of platinum-resistant human ovarian cancer cell lines in comparison to control cell lines was analyzed in vitro. B3CD displayed cell line-specific cytotoxicity screened against a panel of ovarian and other carcinoma cell lines, endothelial and control cells. B3CD, at sub-cytotoxic concentrations, revealed stronger effects on the proliferation of SKOV-3 ovarian cancer cells vs. primary fibroblasts as determined by BrdU incorporation analysis. Treatment with B3CD at 0.5 microM resulted in highly condensed chromatin and fragmented nuclei in SKOV-3 cells but not in primary fibroblasts. B3CD induced cell death at low drug concentrations (< or = 0.5 microM) in SKOV-3 ovarian cancer cells is mediated by the p38 MAPK signaling pathway: B3CD induced p38 MAPK expression and activation in SKOV-3 cells and inhibition of p38 signaling counteracted B3CD induced cell death in vitro. An ovarian cancer cell animal model (human SKOV-3 cell derived xenografts in nude mice) revealed that tumor growth in few B3CD treated mice accelerated while the majority of B3CD treated mice displayed delayed tumor growth or full tumor regression. B3CD possesses anti-ovarian cancer properties in vitro and in vivo. We propose the further development of non-calcemic bromoacetoxy derivatives of vitamin D(3) as potential anti-cancer therapeutics.
Conflict of interest statement
Figures

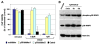

Similar articles
-
Chemotherapeutic effect of calcidiol derivative B3CD in a neuroblastoma xenograft model.Chem Biol Drug Des. 2010 Aug;76(2):164-73. doi: 10.1111/j.1747-0285.2010.00988.x. Epub 2010 May 11. Chem Biol Drug Des. 2010. PMID: 20492445 Free PMC article.
-
Anti-proliferative and pro-apoptotic properties of 3-bromoacetoxy calcidiol in high-risk neuroblastoma.Chem Biol Drug Des. 2007 Oct;70(4):302-10. doi: 10.1111/j.1747-0285.2007.00567.x. Chem Biol Drug Des. 2007. PMID: 17937776 Free PMC article.
-
Iron(III)-salophene: an organometallic compound with selective cytotoxic and anti-proliferative properties in platinum-resistant ovarian cancer cells.PLoS One. 2008 May 28;3(5):e2303. doi: 10.1371/journal.pone.0002303. PLoS One. 2008. PMID: 18509533 Free PMC article.
-
The latest animal models of ovarian cancer for novel drug discovery.Expert Opin Drug Discov. 2018 Mar;13(3):249-257. doi: 10.1080/17460441.2018.1426567. Epub 2018 Jan 17. Expert Opin Drug Discov. 2018. PMID: 29338446 Free PMC article. Review.
-
Patient-derived tumor models are attractive tools to repurpose drugs for ovarian cancer treatment: pre-clinical updates.Oncotarget. 2022 Mar 24;13:553-575. doi: 10.18632/oncotarget.28220. eCollection 2022. Oncotarget. 2022. PMID: 35359749 Free PMC article. Review.
Cited by
-
1α,25(OH)₂D₃ Suppresses the Migration of Ovarian Cancer SKOV-3 Cells through the Inhibition of Epithelial-Mesenchymal Transition.Int J Mol Sci. 2016 Aug 19;17(8):1285. doi: 10.3390/ijms17081285. Int J Mol Sci. 2016. PMID: 27548154 Free PMC article.
-
Chemotherapeutic effect of calcidiol derivative B3CD in a neuroblastoma xenograft model.Chem Biol Drug Des. 2010 Aug;76(2):164-73. doi: 10.1111/j.1747-0285.2010.00988.x. Epub 2010 May 11. Chem Biol Drug Des. 2010. PMID: 20492445 Free PMC article.
-
A coumarin derivative (RKS262) inhibits cell-cycle progression, causes pro-apoptotic signaling and cytotoxicity in ovarian cancer cells.Invest New Drugs. 2011 Feb;29(1):63-72. doi: 10.1007/s10637-009-9335-4. Epub 2009 Oct 29. Invest New Drugs. 2011. PMID: 19865799 Free PMC article.
-
Vitamin D3 stimulates embryonic stem cells but inhibits migration and growth of ovarian cancer and teratocarcinoma cell lines.J Ovarian Res. 2016 Apr 18;9:26. doi: 10.1186/s13048-016-0235-x. J Ovarian Res. 2016. PMID: 27091127 Free PMC article.
-
Vitamin D receptor polymorphisms and prognosis of patients with epithelial ovarian cancer.Br J Cancer. 2009 Dec 15;101(12):1957-60. doi: 10.1038/sj.bjc.6605414. Epub 2009 Nov 10. Br J Cancer. 2009. PMID: 19904266 Free PMC article.
References
-
- Heintz APM, Odicino F, Maisonneuve P, Beller U, Benedet JL, Creasman WT, Ngan HYS, Pecorelli S. International federation of gynecology and obstetrics 25th annual report. Carcinoma of the ovary. Int J Gyn Obst. 2008;83:135–137.
-
- Cancer Facts and Figures. 2008. http://www.cancer.org/downloads/STT/2008CAFFfinalsecured.pdf.
-
- Piccart MJ, Bertelsen K, James K, Cassidy J, Mangioni C, Simonsen E, Stuart G, Kaye S, Vergote I, Blom R, Grimshaw R, Atkinson RJ, Swenerton KD, Trope C, Nardi M, Kaern J, Tumolo S, Timmers P, Roy JA, Lhoas F, Lindvall B, Bacon M, Birt A, Andersen JE, Zee B, Paul J, Baron B, Pecorelli S. Randomized intergroup trial of cisplatin-paclitaxel versus cisplatin-cyclophosphamide in women with advanced epithelial ovarian cancer: three-year results. J Natl Cancer Inst. 2000;92:699–708. - PubMed
-
- McGuire WP, Hoskins WJ, Brady MF, Kucera PR, Partridge EE, Look KY, Clarke-Pearson DL, Davidson M. Cyclophosphamide and cisplatin compared with paclitaxel and cisplatin in patients with stage III and stage IV ovarian cancer. N Engl J Med. 1996;334:1–6. - PubMed
-
- McGuire WP, Ozols RF. Chemotherapy of advanced ovarian cancer. Semin Oncol. 1998;25:340–348. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

