The impact of biomechanics in tissue engineering and regenerative medicine
- PMID: 19583462
- PMCID: PMC3192183
- DOI: 10.1089/ten.TEB.2009.0340
The impact of biomechanics in tissue engineering and regenerative medicine
Abstract
Biomechanical factors profoundly influence the processes of tissue growth, development, maintenance, degeneration, and repair. Regenerative strategies to restore damaged or diseased tissues in vivo and create living tissue replacements in vitro have recently begun to harness advances in understanding of how cells and tissues sense and adapt to their mechanical environment. It is clear that biomechanical considerations will be fundamental to the successful development of clinical therapies based on principles of tissue engineering and regenerative medicine for a broad range of musculoskeletal, cardiovascular, craniofacial, skin, urinary, and neural tissues. Biomechanical stimuli may in fact hold the key to producing regenerated tissues with high strength and endurance. However, many challenges remain, particularly for tissues that function within complex and demanding mechanical environments in vivo. This paper reviews the present role and potential impact of experimental and computational biomechanics in engineering functional tissues using several illustrative examples of past successes and future grand challenges.
Figures
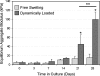




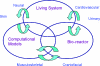
References
-
- Carrel A. Lindberg C. The Culture of Organs. The Canadian Medical Association Journal. New York: Paul B. Hoeber, Inc., Medical Book Department of Harper & Brothers; 1938.
-
- Skalak R. Fox C. NSF Workshop: UCLA Symposia on Molecular and Cellular Biology. Molecular and Cellular Biology, New Series. New York: Alan R. Liss, Inc.; 1988.
-
- Butler D.L. Goldstein S.A. Guilak F. Functional tissue engineering: the role of biomechanics. J Biomech Eng. 2000;122:570. - PubMed
-
- Engler A.J. Rehfeldt F. Sen S. Discher D.E. Microtissue elasticity: measurements by atomic force microscopy and its influence on cell differentiation. Methods Cell Biol. 2007;83:521. - PubMed
-
- Engler A.J. Sen S. Sweeney H.L. Discher D.E. Matrix elasticity directs stem cell lineage specification. Cell. 2006;126:677. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

