Human RPE65 gene therapy for Leber congenital amaurosis: persistence of early visual improvements and safety at 1 year
- PMID: 19583479
- PMCID: PMC2829287
- DOI: 10.1089/hum.2009.086
Human RPE65 gene therapy for Leber congenital amaurosis: persistence of early visual improvements and safety at 1 year
Abstract
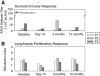
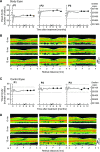
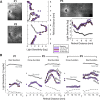
Human gene therapy with rAAV2-vector was performed for the RPE65 form of childhood blindness called Leber congenital amaurosis. In three contemporaneous studies by independent groups, the procedure was deemed safe and there was evidence of visual gain in the short term. At 12 months after treatment, our young adult subjects remained healthy and without vector-related serious adverse events. Results of immunological assays to identify reaction to AAV serotype 2 capsid were unchanged from baseline measurements. Results of clinical eye examinations of study and control eyes, including visual acuities and central retinal structure by in vivo microscopy, were not different from those at the 3-month time point. The remarkable improvements in visual sensitivity we reported by 3 months were unchanged at 12 months. The retinal extent and magnitude of rod and cone components of the visual sensitivity between 3 and 12 months were also the same. The safety and efficacy of human retinal gene transfer with rAAV2-RPE65 vector extends to at least 1 year posttreatment.
Figures
References
-
- Acland G.M. Aguirre G.D. Bennett J. Aleman T.S. Cideciyan A.V. Bennicelli J. Dejneka N.S. Pearce-Kelling S.E. Maguire A.M. Palczewski K. Hauswirth W.W. Jacobson S.G. Long-term restoration of rod and cone vision by single dose rAAV-mediated gene transfer to the retina in a canine model of childhood blindness. Mol. Ther. 2005;12:1072–1082. - PMC - PubMed
-
- Aguirre G.K. Komáromy A.M. Cideciyan A.V. Brainard D.H. Aleman T.S. Roman A.J. Avants B.B. Gee J.C. Korczykowski M. Hauswirth W.W. Acland G.M. Aguirre G.D. Jacobson S.G. Canine and human visual cortex intact and responsive despite early retinal blindness from RPE65 mutation. PLoS Med. 2007;4:e230. - PMC - PubMed
-
- Aleman T.S. Cideciyan A.V. Sumaroka A. Windsor E.A. Herrera W. White D.A. Kaushal S. Naidu A. Roman A.J. Schwartz S.B. Stone E.M. Jacobson S.G. Retinal laminar architecture in human retinitis pigmentosa caused by rhodopsin gene mutations. Invest. Ophthalmol. Vis. Sci. 2008;49:1580–1590. - PMC - PubMed
-
- Bainbridge J.W. Smith A.J. Barker S.S. Robbie S. Henderson R. Balaggan K. Viswanathan A. Holder G.E. Stockman A. Tyler N. Petersen-Jones S. Bhattacharya S.S. Thrasher A.J. Fitzke F.W. Carter B.J. Rubin G.S. Moore A.T. Ali R.R. Effect of gene therapy on visual function in Leber's congenital amaurosis. N. Engl. J. Med. 2008;358:2231–2239. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

