Histone deacetylase inhibition and the regulation of cell growth with particular reference to liver pathobiology
- PMID: 19583816
- PMCID: PMC4516460
- DOI: 10.1111/j.1582-4934.2009.00831.x
Histone deacetylase inhibition and the regulation of cell growth with particular reference to liver pathobiology
Abstract
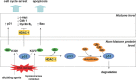
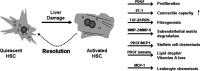
The transcriptional activity of genes largely depends on the accessibility of specific chromatin regions to transcriptional regulators. This process is controlled by diverse post-transcriptional modifications of the histone amino termini of which reversible acetylation plays a vital role. Histone acetyltransferases (HATs) are responsible for the addition of acetyl groups and histone deacetylases (HDACs) catalyse the reverse reaction. In general, though not exclusively, histone acetylation is associated with a positive regulation of transcription, whereas histone deacetylation is correlated with transcriptional silencing. The elucidation of unequivocal links between aberrant action of HDACs and tumorigenesis lies at the base of key scientific importance of these enzymes. In particular, the potential benefit of HDAC inhibition has been confirmed in various tumour cell lines, demonstrating antiproliferative, differentiating and pro-apoptotic effects. Consequently, the dynamic quest for HDAC inhibitors (HDIs) as a new class of anticancer drugs was set off, resulting in a number of compounds that are currently evaluated in clinical trials. Ironically, the knowledge with respect to the expression pattern and function of individual HDAC isoenzymes remains largely elusive. In the present review, we provide an update of the current knowledge on the involvement of HDACs in the regulation of fundamental cellular processes in the liver, being the main site for drug metabolism within the body. Focus lies on the involvement of HDACs in the regulation of growth of normal and transformed hepatocytes and the transdifferentiation process of stellate cells. Furthermore, extrapolation of our present knowledge on HDAC functionality towards innovative treatment of malignant and non-malignant, hyperproliferative and inflammatory disorders is discussed.
Figures

References
-
- Sandman K, Reeve JN. Archaeal histones and the origin of the histone fold. Curr Opin Microbiol. 2006;9:520–5. - PubMed
-
- Strahl BD, Allis CD. The language of covalent histone modifications. Nature. 2000;403:41–5. - PubMed
-
- Luger K. Structure and dynamic behavior of nucleosomes. Curr Opin Genet Dev. 2003;13:127–35. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

