A new homogeneous high-throughput screening assay for profiling compound activity on the human ether-a-go-go-related gene channel
- PMID: 19583963
- PMCID: PMC2766802
- DOI: 10.1016/j.ab.2009.07.003
A new homogeneous high-throughput screening assay for profiling compound activity on the human ether-a-go-go-related gene channel
Abstract
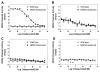
Long QT syndrome, either inherited or acquired from drug treatments, can result in ventricular arrhythmia (torsade de pointes) and sudden death. Human ether-a-go-go-related gene (hERG) channel inhibition by drugs is now recognized as a common reason for the acquired form of long QT syndrome. It has been reported that more than 100 known drugs inhibit the activity of the hERG channel. Since 1997, several drugs have been withdrawn from the market due to the long QT syndrome caused by hERG inhibition. Food and Drug Administration regulations now require safety data on hERG channels for investigative new drug (IND) applications. The assessment of compound activity on the hERG channel has now become an important part of the safety evaluation in the process of drug discovery. During the past decade, several in vitro assay methods have been developed and significant resources have been used to characterize hERG channel activities. However, evaluation of compound activities on hERG have not been performed for large compound collections due to technical difficulty, lack of throughput, and/or lack of biological relevance to function. Here we report a modified form of the FluxOR thallium flux assay, capable of measuring hERG activity in a homogeneous 1536-well plate format. To validate the assay, we screened a 7-point dilution series of the LOPAC 1280 library collection and reported rank order potencies of ten common hERG inhibitors. A correlation was also observed for the hERG channel activities of 10 known hERG inhibitors determined in this thallium flux assay and in the patch clamp experiment. Our findings indicate that this thallium flux assay can be used as an alternative method to profile large-volume compound libraries for compound activity on the hERG channel.
Figures




References
-
- Ganetzky B, Wu CF. Neurogenetic analysis of potassium currents in Drosophila: synergistic effects on neuromuscular transmission in double mutants. J Neurogenet. 1983;1:17–28. - PubMed
-
- Wu CF, Ganetzky B, Haugland FN, Liu AX. Potassium currents in Drosophila: different components affected by mutations of two genes. Science. 1983;220:1076–1078. - PubMed
-
- Warmke J, Drysdale R, Ganetzky B. A distinct potassium channel polypeptide encoded by the Drosophila eag locus. Science. 1991;252:1560–1562. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

