Human antibody titers to Epstein-Barr Virus (EBV) gp350 correlate with neutralization of infectivity better than antibody titers to EBV gp42 using a rapid flow cytometry-based EBV neutralization assay
- PMID: 19584018
- PMCID: PMC2728425
- DOI: 10.1016/j.virol.2009.06.013
Human antibody titers to Epstein-Barr Virus (EBV) gp350 correlate with neutralization of infectivity better than antibody titers to EBV gp42 using a rapid flow cytometry-based EBV neutralization assay
Abstract
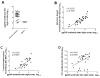
Measurement of neutralizing antibodies to Epstein-Barr virus (EBV) is important for evaluation of candidate vaccines. The current neutralization assay is based on antibody inhibition of EBV transformation of B cells and requires 6 weeks to perform. We developed a rapid, quantitative flow cytometry assay and show that neutralizing antibody titers measured by the new assay strongly correlate with antibody titers in the standard transformation-based assay. Antibodies to EBV gp350 and gp42 have been shown to block infection of B cells by EBV. Using new assays to quantify antibodies to these glycoproteins, we show for the first time that human plasma contains high titers of antibody to gp42; these titers correlate with neutralization of EBV infectivity or transformation. Furthermore, we show that antibody titers to EBV gp350 correlate more strongly with neutralization than antibody titers to gp42. These assays should be useful in accessing antibody responses to candidate EBV vaccines.
Figures
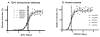
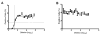
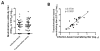
Similar articles
-
High Epstein-Barr Virus Load and Genomic Diversity Are Associated with Generation of gp350-Specific Neutralizing Antibodies following Acute Infectious Mononucleosis.J Virol. 2016 Dec 16;91(1):e01562-16. doi: 10.1128/JVI.01562-16. Print 2017 Jan 1. J Virol. 2016. PMID: 27733645 Free PMC article.
-
Association between Antibody Responses to Epstein-Barr Virus Glycoproteins, Neutralization of Infectivity, and the Risk of Nasopharyngeal Carcinoma.mSphere. 2020 Dec 2;5(6):e00901-20. doi: 10.1128/mSphere.00901-20. mSphere. 2020. PMID: 33268566 Free PMC article.
-
Epstein-Barr virus gp350-specific antibody titers and antibody-dependent cellular cytotoxic effector function in different groups of patients: a study using cloned gp350-expressing transfected human T cell targets.J Infect Dis. 1994 Dec;170(6):1439-47. doi: 10.1093/infdis/170.6.1439. J Infect Dis. 1994. PMID: 7995983
-
Development of a robust, higher throughput green fluorescent protein (GFP)-based Epstein-Barr Virus (EBV) micro-neutralization assay.J Virol Methods. 2017 Sep;247:15-21. doi: 10.1016/j.jviromet.2017.04.012. Epub 2017 Apr 27. J Virol Methods. 2017. PMID: 28457783
-
Epstein Barr Virus: Development of Vaccines and Immune Cell Therapy for EBV-Associated Diseases.Front Immunol. 2021 Oct 8;12:734471. doi: 10.3389/fimmu.2021.734471. eCollection 2021. Front Immunol. 2021. PMID: 34691042 Free PMC article. Review.
Cited by
-
Immunogenic particles with a broad antigenic spectrum stimulate cytolytic T cells and offer increased protection against EBV infection ex vivo and in mice.PLoS Pathog. 2018 Dec 6;14(12):e1007464. doi: 10.1371/journal.ppat.1007464. eCollection 2018 Dec. PLoS Pathog. 2018. PMID: 30521644 Free PMC article.
-
Immunization with a self-assembling nanoparticle vaccine displaying EBV gH/gL protects humanized mice against lethal viral challenge.Cell Rep Med. 2022 Jun 21;3(6):100658. doi: 10.1016/j.xcrm.2022.100658. Epub 2022 Jun 14. Cell Rep Med. 2022. PMID: 35705092 Free PMC article.
-
A Pentavalent Epstein-Barr Virus-Like Particle Vaccine Elicits High Titers of Neutralizing Antibodies against Epstein-Barr Virus Infection in Immunized Rabbits.Vaccines (Basel). 2020 Apr 6;8(2):169. doi: 10.3390/vaccines8020169. Vaccines (Basel). 2020. PMID: 32268575 Free PMC article.
-
Immunization With Fc-Based Recombinant Epstein-Barr Virus gp350 Elicits Potent Neutralizing Humoral Immune Response in a BALB/c Mice Model.Front Immunol. 2018 May 1;9:932. doi: 10.3389/fimmu.2018.00932. eCollection 2018. Front Immunol. 2018. PMID: 29765376 Free PMC article.
-
Identification of multiple potent neutralizing and non-neutralizing antibodies against Epstein-Barr virus gp350 protein with potential for clinical application and as reagents for mapping immunodominant epitopes.Virology. 2019 Oct;536:1-15. doi: 10.1016/j.virol.2019.07.026. Epub 2019 Jul 30. Virology. 2019. PMID: 31377598 Free PMC article.
References
-
- Andrewes CH, Elford WJ. Observations on anti-phage sera. I: The percentage law. Br J Exp Pathol. 1933;14:367–376.
-
- Biacchesi S, Skiadopoulos MH, Yang L, Murphy BR, Collins PL, Buchholz UJ. Rapid human metapneumovirus microneutralization assay based on green fluorescent protein expression. J Virol Methods. 2005;128:192–197. - PubMed
-
- Borza CM, Hutt-Fletcher LM. Alternate replication in B cells and epithelial cells switches tropism of Epstein-Barr virus. Nat Med. 2002;8:594–599. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

