Nuclear factor-kappaB regulates estrogen receptor-alpha transcription in the human heart
- PMID: 19584059
- PMCID: PMC2757174
- DOI: 10.1074/jbc.M109.000463
Nuclear factor-kappaB regulates estrogen receptor-alpha transcription in the human heart
Abstract
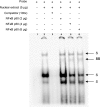
Estrogen receptor (ER)-mediated effects have been associated with the modulation of myocardial hypertrophy in animal models and in humans, but the regulation of ER expression in the human heart has not yet been analyzed. In various cell lines and tissues, multiple human estrogen receptor alpha (hERalpha) mRNA isoforms are transcribed from distinct promoters and differ in their 5'-untranslated regions. Using PCR-based strategies, we show that in the human heart the ERalpha mRNA is transcribed from multiple promoters, namely, A, B, C, and F, of which the F-promoter is most frequently used variant. Transient transfection reporter assays in a human cardiac myocyte cell line (AC16) with F-promoter deletion constructs demonstrated a negative regulatory region within this promoter. Site-directed mutagenesis and electrophoretic mobility shift assays indicated that NF-kappaB binds to this region. An inhibition of NF-kappaB activity by parthenolide significantly increased the transcriptional activity of the F-promoter. Increasing NF-kappaB expression by tumor necrosis factor-alpha reduced the expression of ERalpha, indicating that the NF-kappaB pathway inhibits expression of ERalpha in human cardiomyocytes. Finally, 17beta-estradiol induced the transcriptional activity of hERalpha promoters A, B, C, and F. In conclusion, inflammatory stimuli suppress hERalpha expression via activation and subsequent binding of NF-kappaB to the ERalpha F-promoter, and 17beta-estradiol/hERalpha may antagonize the inhibitory effect of NF-kappaB. This suggests interplay between estrogen/estrogen receptors and the pro-hypertrophic and inflammatory responses to NF-kappaB.
Figures


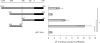

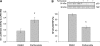


Similar articles
-
The interplay between NF-kappaB and E2F1 coordinately regulates inflammation and metabolism in human cardiac cells.PLoS One. 2011;6(5):e19724. doi: 10.1371/journal.pone.0019724. Epub 2011 May 23. PLoS One. 2011. PMID: 21625432 Free PMC article.
-
17beta-estradiol inhibits the production of RANTES in human keratinocytes.J Invest Dermatol. 2003 Mar;120(3):420-7. doi: 10.1046/j.1523-1747.2003.12067.x. J Invest Dermatol. 2003. PMID: 12603855
-
The classical and a non-classical pathways associated with NF-kappaB are involved in estrogen-mediated regulation of calbindin-D9k gene in rat pituitary cells.Mol Cell Endocrinol. 2007 Oct 15;277(1-2):42-50. doi: 10.1016/j.mce.2007.07.009. Epub 2007 Aug 2. Mol Cell Endocrinol. 2007. PMID: 17825480
-
Determining the transrepression activity of xenoestrogen on nuclear factor-kappa B in Cos-1 cells by estrogen receptor-alpha.Int J Toxicol. 2007 Sep-Oct;26(5):441-9. doi: 10.1080/10915810701620317. Int J Toxicol. 2007. PMID: 17963131
-
Domains of estrogen receptor alpha (ERalpha) required for ERalpha/Sp1-mediated activation of GC-rich promoters by estrogens and antiestrogens in breast cancer cells.Mol Endocrinol. 2003 May;17(5):804-17. doi: 10.1210/me.2002-0406. Epub 2003 Feb 6. Mol Endocrinol. 2003. PMID: 12576490
Cited by
-
Age-related decline in murine heart and skeletal muscle performance is attenuated by reduced Ahnak1 expression.J Cachexia Sarcopenia Muscle. 2021 Oct;12(5):1249-1265. doi: 10.1002/jcsm.12749. Epub 2021 Jul 1. J Cachexia Sarcopenia Muscle. 2021. PMID: 34212535 Free PMC article.
-
Sex-Based Mechanisms of Cardiac Development and Function: Applications for Induced-Pluripotent Stem Cell Derived-Cardiomyocytes.Int J Mol Sci. 2024 May 29;25(11):5964. doi: 10.3390/ijms25115964. Int J Mol Sci. 2024. PMID: 38892161 Free PMC article. Review.
-
Triphenyl phosphate activates estrogen receptor α/NF-κB/ cyclin D1 signaling to stimulate cell cycle progression in human Ishikawa endometrial cancer cells.Obstet Gynecol Sci. 2022 Nov;65(6):531-541. doi: 10.5468/ogs.22108. Epub 2022 Aug 4. Obstet Gynecol Sci. 2022. PMID: 36254600 Free PMC article.
-
17beta-Estradiol inhibits matrix metalloproteinase-2 transcription via MAP kinase in fibroblasts.Cardiovasc Res. 2010 Mar 1;85(4):719-28. doi: 10.1093/cvr/cvp350. Epub 2009 Oct 27. Cardiovasc Res. 2010. PMID: 19861308 Free PMC article.
-
Evaluation of activity of an estrogen-derivative as cardioprotector drug using an ischemia-reperfusion injury model.Int J Clin Exp Med. 2015 Aug 15;8(8):12041-55. eCollection 2015. Int J Clin Exp Med. 2015. PMID: 26550116 Free PMC article.
References
-
- Mendelsohn M. E., Karas R. H. (2005) Science 308, 1583–1587 - PubMed
-
- Karas R. H., Patterson B. L., Mendelsohn M. E. (1994) Circulation 89, 1943–1950 - PubMed
-
- Venkov C. D., Rankin A. B., Vaughan D. E. (1996) Circulation 94, 727–733 - PubMed
-
- Nordmeyer J., Eder S., Mahmoodzadeh S., Martus P., Fielitz J., Bass J., Bethke N., Zurbrügg H. R., Pregla R., Hetzer R., Regitz-Zagrosek V. (2004) Circulation 110, 3270–3275 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

