Foxo1 links hyperglycemia to LDL oxidation and endothelial nitric oxide synthase dysfunction in vascular endothelial cells
- PMID: 19584310
- PMCID: PMC2750207
- DOI: 10.2337/db09-0167
Foxo1 links hyperglycemia to LDL oxidation and endothelial nitric oxide synthase dysfunction in vascular endothelial cells
Abstract
Objective: Atherosclerotic cardiovascular disease is the leading cause of death among people with diabetes. Generation of oxidized LDLs and reduced nitric oxide (NO) availability because of endothelial NO synthase (eNOS) dysfunction are critical events in atherosclerotic plaque formation. Biochemical mechanism leading from hyperglycemia to oxLDL formation and eNOS dysfunction is unknown.
Research design and methods: We show that glucose, acting through oxidative stress, activates the transcription factor Foxo1 in vascular endothelial cells.
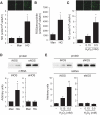
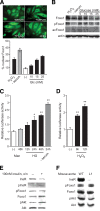
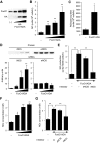
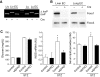
Results: Foxo1 promotes inducible NOS (iNOS)-dependent NO-peroxynitrite generation, which leads in turn to LDL oxidation and eNOS dysfunction. We demonstrate that Foxo1 gain-of-function mimics the effects of hyperglycemia on this process, whereas conditional Foxo1 knockout in vascular endothelial cells prevents it.
Conclusions: The findings reveal a hitherto unsuspected role of the endothelial iNOS-NO-peroxynitrite pathway in lipid peroxidation and eNOS dysfunction and suggest that Foxo1 activation in response to hyperglycemia brings about proatherogenic changes in vascular endothelial cell function.
Figures




References
-
- National Institute of Diabetes and Digestive and Kidney Diseases. National Diabetes Statistics Fact Sheet: General Information and National Estimates on Diabetes in the United States Bethesda, MD, U.S. Department of Health and Human Services, National Institute of Health, 2005
-
- U.K. Prospective Diabetes Study Group. Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). Lancet 1998; 352: 837– 853 - PubMed
-
- Hanley AJ, Williams K, Stern MP, Haffner SM: Homeostasis model assessment of insulin resistance in relation to the incidence of cardiovascular disease: the San Antonio Heart Study. Diabetes Care 2002; 25: 1177– 1184 - PubMed
-
- Yip J, Facchini FS, Reaven GM: Resistance to insulin-mediated glucose disposal as a predictor of cardiovascular disease. J Clin Endocrinol Metab 1998; 83: 2773– 2776 - PubMed
-
- Brownlee M: Biochemistry and molecular cell biology of diabetic complications. Nature 2001; 414: 813– 820 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

