Vascular extracellular matrix and arterial mechanics
- PMID: 19584318
- PMCID: PMC2775470
- DOI: 10.1152/physrev.00041.2008
Vascular extracellular matrix and arterial mechanics
Abstract
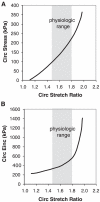
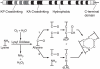
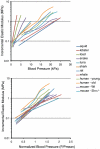
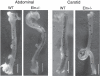
An important factor in the transition from an open to a closed circulatory system was a change in vessel wall structure and composition that enabled the large arteries to store and release energy during the cardiac cycle. The component of the arterial wall in vertebrates that accounts for these properties is the elastic fiber network organized by medial smooth muscle. Beginning with the onset of pulsatile blood flow in the developing aorta, smooth muscle cells in the vessel wall produce a complex extracellular matrix (ECM) that will ultimately define the mechanical properties that are critical for proper function of the adult vascular system. This review discusses the structural ECM proteins in the vertebrate aortic wall and will explore how the choice of ECM components has changed through evolution as the cardiovascular system became more advanced and pulse pressure increased. By correlating vessel mechanics with physiological blood pressure across animal species and in mice with altered vessel compliance, we show that cardiac and vascular development are physiologically coupled, and we provide evidence for a universal elastic modulus that controls the parameters of ECM deposition in vessel wall development. We also discuss mechanical models that can be used to design better tissue-engineered vessels and to test the efficacy of clinical treatments.
Figures







References
-
- Abrams WR, Ma RI, Kucich U, Bashir MM, Decker S, Tsipouras P, McPherson JD, Wasmuth JJ, Rosenbloom J. Molecular cloning of the microfibrillar protein MFAP3 and assignment to the gene to human chromosome 5q32-q33.2. Genomics. 1995;26:47–54. - PubMed
-
- Ahimastos AA, Natoli AK, Lawler A, Blombery PA, Kingwell BA. Ramipril reduces large-artery stiffness in peripheral arterial disease and promotes elastogenic remodeling in cell culture. Hypertension. 2005;45:1194–1199. - PubMed
-
- Arciniegas E, Neves CY, Candelle D, Parada D. Differential versican isoforms and aggrecan expression in the chicken embryo aorta. Anat Rec A Discov Mol Cell Evol Biol. 2004;279:592–600. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

