A risk-adapted, response-based approach using ABVE-PC for children and adolescents with intermediate- and high-risk Hodgkin lymphoma: the results of P9425
- PMID: 19584400
- PMCID: PMC2744567
- DOI: 10.1182/blood-2008-10-184143
A risk-adapted, response-based approach using ABVE-PC for children and adolescents with intermediate- and high-risk Hodgkin lymphoma: the results of P9425
Erratum in
-
Erratum: Schwartz CL, Constine LS, Villaluna D, et al. A risk-adapted, response-based approach using ABVE-PC for children and adolescents with intermediate- and high-risk Hodgkin lymphoma: the results of P9425. Blood. 2009;114(10):2051-2059.Blood. 2016 Jul 28;128(4):605. doi: 10.1182/blood-2016-06-723056. Blood. 2016. PMID: 31265504 Free PMC article.
Abstract
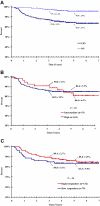
Current treatment strategies for Hodgkin lymphoma result in excellent survival but often confer significant long-term toxicity. We designed ABVE-PC (doxorubicin, bleomycin, vincristine, etoposide, prednisone, cyclophosphamide) to (1) enhance treatment efficacy by dose-dense drug delivery and (2) reduce risk of long-term sequelae by response-based reduction of cumulative chemotherapy. Efficient induction of early response by dose-dense drug delivery supported an early-response-adapted therapeutic paradigm. The 216 eligible patients were younger than 22 years with intermediate- or high-risk Hodgkin lymphoma. ABVE-PC was administered every 21 days. Rapid early responders (RERs) to 3 ABVE-PC cycles received 21 Gy radiation to involved regions; RER was documented in 63% of patients. Slow early responders received 2 additional ABVE-PC cycles before 21 Gy radiation. Five-year event-free-survival was 84%: 86% for the RER and 83% for the slow early responders (P = .85). Only 1% of patients had progressive disease. Five-year overall survival was 95%. With this regimen, cumulative doses of alkylators, anthracyclines, and epipodophyllotoxins are below thresholds usually associated with significant long-term toxicity. ABVE-PC is a dose-dense regimen that provides outstanding event-free survival/overall survival with short duration, early-response-adapted therapy.
Trial registration: ClinicalTrials.gov NCT00005578.
Figures

References
-
- DeVita VT, Jr, Simon RM, Hubbard SM, et al. Curability of advanced Hodgkin's disease with chemotherapy: long-term follow-up of MOPP-treated patients at the National Cancer Institute. Ann Intern Med. 1980;92(5):587–595. - PubMed
-
- Santoro A, Bonadonna G, Bonfante V, et al. Alternating drug combinations in the treatment of advanced Hodgkin's disease. N Engl J Med. 1982;306(13):770–775. - PubMed
-
- Longo DL, Young RC, Wesley M, et al. Twenty years of MOPP therapy for Hodgkin's disease. J Clin Oncol. 1986;4(9):1295–1306. - PubMed
-
- Van Rijswijk RE, Haanen C, Dekker AW, et al. Dose intensity of MOPP chemotherapy and survival in Hodgkin's disease. J Clin Oncol. 1989;7(12):1776–1782. - PubMed
-
- Carde P, MacKintosh FR, Rosenberg SA. A dose and time response analysis of the treatment of Hodgkin's disease with MOPP chemotherapy. J Clin Oncol. 1983;1(2):146–153. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

