Using social networks to recruit an HIV vaccine preparedness cohort
- PMID: 19584741
- PMCID: PMC2783777
- DOI: 10.1097/QAI.0b013e3181acff91
Using social networks to recruit an HIV vaccine preparedness cohort
Abstract
Objectives: To evaluate a social network approach to develop an adolescent cohort for HIV vaccine preparedness and investigate characteristics that influence recruitment.
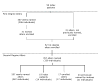
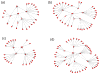
Methods: We summarize baseline data from a prospective cohort study that included 4 sessions over 6 months. Fifty-nine HIV-infected adolescent and adult patients of a family-based HIV clinic named significant others and indicated willingness to involve them in this study. Sixty-two adolescent and adult significant others not known to be HIV infected were enrolled. Logistic regression was used to estimate factors associated with willingness.
Results: Participants identified 624 social network members including 276 adolescents (44%). Network member's awareness of the index's HIV positivity (P < 0.01) and older age (P = 0.05) affected willingness. Respondents were less willing to invite drug-risk alters (P = 0.006). Adolescents were willing to invite more adolescents than were adults (P < 0.0001). Adolescents younger than 18 years old reported fewer sexual and drug-using risk behaviors than expected.
Conclusions: HIV-infected patients are willing to recruit their social networks, provided concerns about disclosure of HIV status are addressed. Using social networks to identify and recruit adolescent populations is appropriate and feasible for vaccine preparedness activities, future vaccine trials, and other prevention programs, but procedures are needed to selectively identify and retain high-risk youth.
Figures
References
-
- Coletti AS, Heagerty P, Sheon AR, et al. Randomized, controlled evaluation of a prototype informed consent process for HIV vaccine efficacy trials. J Acquir Immune Defic Syndr. 2003;32(2):161–9. - PubMed
-
- Buchbinder SP, Metch B, Holte SE, et al. Determinants of enrollment in a preventive HIV vaccine trial: Hypothetical versus actual willingness and barriers to participation. J Acquir Immune Defic Syndr. 2004;36(1):604–12. - PubMed
-
- Bekker LG, Jaspan H, McIntyre J, et al. Adolescents and HIV vaccine trials: What are the clinical trial site issues? J Int Assoc Physicians AIDS Care. 2005;4(4):93–7. - PubMed
-
- Stanford PD, Monte DA, Briggs FM, et al. Recruitment and Retention of Adolescent Participants in HIV Research: Findings from the REACH (Reaching for Excellence in Adolescent Care and Health) Project. J Adolesc Health. 2003;32(3):192–203. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

