Elucidating regulatory mechanisms downstream of a signaling pathway using informative experiments
- PMID: 19584836
- PMCID: PMC2724975
- DOI: 10.1038/msb.2009.45
Elucidating regulatory mechanisms downstream of a signaling pathway using informative experiments
Abstract
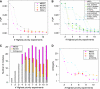
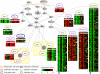
Signaling cascades are triggered by environmental stimulation and propagate the signal to regulate transcription. Systematic reconstruction of the underlying regulatory mechanisms requires pathway-targeted, informative experimental data. However, practical experimental design approaches are still in their infancy. Here, we propose a framework that iterates design of experiments and identification of regulatory relationships downstream of a given pathway. The experimental design component, called MEED, aims to minimize the amount of laboratory effort required in this process. To avoid ambiguity in the identification of regulatory relationships, the choice of experiments maximizes diversity between expression profiles of genes regulated through different mechanisms. The framework takes advantage of expert knowledge about the pathways under study, formalized in a predictive logical model. By considering model-predicted dependencies between experiments, MEED is able to suggest a whole set of experiments that can be carried out simultaneously. Our framework was applied to investigate interconnected signaling pathways in yeast. In comparison with other approaches, MEED suggested the most informative experiments for unambiguous identification of transcriptional regulation in this system.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures




References
-
- Akutsu T, Kuhara S, Maruyama O, Miyano S (1998) A system for identifying genetic networks from gene expression patterns produced by gene disruptions and overexpressions. Genome Inform Ser Workshop Genome Inform 9: 151–160 - PubMed
-
- Ashburner M, Ball CA, Blake JA, Botstein D, Butler H, Cherry JM, Davis AP, Dolinski K, Dwight SS, Eppig JT, Harris MA, Hill DP, Issel-Tarver L, Kasarskis A, Lewis S, Matese JC, Richardson JE, Ringwald M, Rubin GM, Sherlock G (2000) Gene ontology: tool for the unification of biology. The Gene Ontology Consortium. Nat Genet 25: 25–29 - PMC - PubMed
-
- Bauer S, Grossmann S, Vingron M, Robinson PN (2008) Ontologizer 2.0—a multifunctional tool for GO term enrichment analysis and data exploration. Bioinformatics 24: 1650–1651 - PubMed
-
- Bolouri H, Davidson EH (2002) Modeling transcriptional regulatory networks. Bioessays 24: 1118–1129 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

