Alefacept promotes co-stimulation blockade based allograft survival in nonhuman primates
- PMID: 19584865
- PMCID: PMC2772128
- DOI: 10.1038/nm.1993
Alefacept promotes co-stimulation blockade based allograft survival in nonhuman primates
Abstract
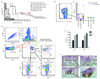
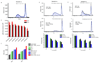
Memory T cells promote allograft rejection particularly in co-stimulation blockade-based immunosuppressive regimens. Here we show that the CD2-specific fusion protein alefacept (lymphocyte function-associated antigen-3-Ig; LFA -3-Ig) selectively eliminates memory T cells and, when combined with a co-stimulation blockade-based regimen using cytotoxic T lymphocyte antigen-4 (CTLA-4)-Ig, a CD80- and CD86-specific fusion protein, prevents renal allograft rejection and alloantibody formation in nonhuman primates. These results support the immediate translation of a regimen for the prevention of allograft rejection without the use of calcineurin inhibitors, steroids or pan-T cell depletion.
Figures
Similar articles
-
Belatacept and sirolimus prolong nonhuman primate renal allograft survival without a requirement for memory T cell depletion.Am J Transplant. 2013 Feb;13(2):320-8. doi: 10.1111/j.1600-6143.2012.04342.x. Epub 2013 Jan 11. Am J Transplant. 2013. PMID: 23311611 Free PMC article.
-
Selective targeting of human alloresponsive CD8+ effector memory T cells based on CD2 expression.Am J Transplant. 2011 Jan;11(1):22-33. doi: 10.1111/j.1600-6143.2010.03317.x. Epub 2010 Nov 10. Am J Transplant. 2011. PMID: 21070604 Free PMC article.
-
Belatacept and sirolimus prolong nonhuman primate islet allograft survival: adverse consequences of concomitant alefacept therapy.Am J Transplant. 2013 Feb;13(2):312-9. doi: 10.1111/j.1600-6143.2012.04341.x. Epub 2012 Dec 27. Am J Transplant. 2013. PMID: 23279640 Free PMC article.
-
Novel immunosuppressive agents in kidney transplantation.Clin Nephrol. 2010 May;73(5):333-43. doi: 10.5414/cnp73333. Clin Nephrol. 2010. PMID: 20420793 Review.
-
Biological agents in kidney transplantation: belatacept is entering the field.Expert Opin Biol Ther. 2010 Oct;10(10):1501-8. doi: 10.1517/14712598.2010.514901. Expert Opin Biol Ther. 2010. PMID: 20726688 Review.
Cited by
-
mTOR Inhibitor Therapy Diminishes Circulating CD8+ CD28- Effector Memory T Cells and Improves Allograft Inflammation in Belatacept-refractory Renal Allograft Rejection.Transplantation. 2020 May;104(5):1058-1069. doi: 10.1097/TP.0000000000002917. Transplantation. 2020. PMID: 31415033 Free PMC article. Clinical Trial.
-
GVHD after haploidentical transplantation: a novel, MHC-defined rhesus macaque model identifies CD28- CD8+ T cells as a reservoir of breakthrough T-cell proliferation during costimulation blockade and sirolimus-based immunosuppression.Blood. 2010 Dec 9;116(24):5403-18. doi: 10.1182/blood-2010-06-289272. Epub 2010 Sep 10. Blood. 2010. PMID: 20833977 Free PMC article.
-
Recognizing Complexity of CD8 T Cells in Transplantation.Transplantation. 2024 Nov 1;108(11):2186-2196. doi: 10.1097/TP.0000000000005001. Epub 2024 Apr 19. Transplantation. 2024. PMID: 38637929 Review.
-
CD57(+) CD4 T Cells Underlie Belatacept-Resistant Allograft Rejection.Am J Transplant. 2016 Apr;16(4):1102-12. doi: 10.1111/ajt.13613. Epub 2016 Jan 14. Am J Transplant. 2016. PMID: 26603381 Free PMC article.
-
B Cell Depletion With an Anti-CD20 Antibody Enhances Alloreactive Memory T Cell Responses After Transplantation.Am J Transplant. 2016 Feb;16(2):672-8. doi: 10.1111/ajt.13483. Epub 2015 Nov 9. Am J Transplant. 2016. PMID: 26552037 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

