All-oral combination of oral vinorelbine and capecitabine as first-line chemotherapy in HER2-negative metastatic breast cancer: an International Phase II Trial
- PMID: 19584872
- PMCID: PMC2720198
- DOI: 10.1038/sj.bjc.6605156
All-oral combination of oral vinorelbine and capecitabine as first-line chemotherapy in HER2-negative metastatic breast cancer: an International Phase II Trial
Abstract
Background: This multicentre, international phase II trial evaluated the efficacy and safety profile of a first-line combination of oral vinorelbine plus capecitabine for women with metastatic breast cancer (MBC).
Methods: Patients with measurable, HER2-negative disease received, as a first line in metastatic setting, 3-weekly cycles of oral vinorelbine 80 mg m(-2) (after a first cycle at 60) on day 1 and day 8, plus capecitabine 1000 mg m(-2) (750 if >or=65 years of age) twice daily, on days 1-14. Treatment was continued until progression or unacceptable toxicity.
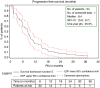
Results: A total of 55 patients were enrolled and 54 were treated (median age: 58.5 years). Most (78%) had visceral involvement and 63% had received earlier (neo)adjuvant chemotherapy. The objective response rate (RECIST) in 49 evaluable patients was 51% (95% confidence interval (CI), 36-66), including complete response in 4%. The clinical benefit rate (response or stable disease for >or=6 months) was 63% (95% CI, 48-77). The median duration of response was 7.2 months (95% CI, 6.4-10.2). After a median follow-up of 41 months, median progression-free survival was 8.4 months (95% CI, 5.8-9.7) and median overall survival was 29.2 months (95% CI, 18.2-40.1). Treatment-related adverse events were manageable, the main grade 3-4 toxicity was neutropaenia (49%); two patients experienced febrile neutropaenia and three patients had a neutropaenic infection (including one septic death). A particularly low rate of alopaecia was observed.
Conclusion: These results show that the all-oral combination of oral vinorelbine and capecitabine is an effective and well-tolerated first-line regimen for MBC.
Figures
References
-
- Albain K, Nag S, Calderillo-Ruiz G, Jordaan J, Llombart A, Pluzanska A, Rolski J, Melemed A, Reyes-Vidal J, Sekhon J, Simms L, O’Shaughnessy J (2008) Gemcitabine plus paclitaxel vs paclitaxel monotherapy in patients with metastatic breast cancer and prior anthracycline treatment. J Clin Oncol 26: 3950–3957 - PubMed
-
- Anton A, Lluch A, Casado A, Provencio M, Munoz M, Lao J, Bermejo B, Paules A, Gayo J, Martin M (2008) Phase I-II study of oral vinorelbine and capecitabine in metastatic breast cancer: results of the phase I trial. J Clin Oncol 26: 67s (abstract 1105) - PubMed
-
- Coates A, Stockler M, Wilcken N (2003) Controversies in Metastatic Breast Cancer: Optimal Duration of Chemotherapy. ASCO Educational Book. pp 119–121
-
- Delcambre C, Veyret C, Levy C, Switsers O, Allouache D, Raban N, Grellard JM, Leconte A, Delozier T (2005) A phase I/II study of capecitabine combined with oral vinorelbine as first or second line therapy in locally advanced or metastatic breast cancer. Breast Cancer Res Treat 94: S67 (abstract 1061)
-
- Ershler W (2006) Capecitabine monotherapy: safe and effective treatment for metastatic breast cancer. Oncologist 11: 325–335 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous