PINP: a new method of tagging neuronal populations for identification during in vivo electrophysiological recording
- PMID: 19584920
- PMCID: PMC2702752
- DOI: 10.1371/journal.pone.0006099
PINP: a new method of tagging neuronal populations for identification during in vivo electrophysiological recording
Abstract
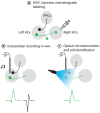
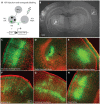
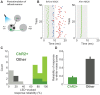
Neural circuits are exquisitely organized, consisting of many different neuronal subpopulations. However, it is difficult to assess the functional roles of these subpopulations using conventional extracellular recording techniques because these techniques do not easily distinguish spikes from different neuronal populations. To overcome this limitation, we have developed PINP (Photostimulation-assisted Identification of Neuronal Populations), a method of tagging neuronal populations for identification during in vivo electrophysiological recording. The method is based on expressing the light-activated channel channelrhodopsin-2 (ChR2) to restricted neuronal subpopulations. ChR2-tagged neurons can be detected electrophysiologically in vivo since illumination of these neurons with a brief flash of blue light triggers a short latency reliable action potential. We demonstrate the feasibility of this technique by expressing ChR2 in distinct populations of cortical neurons using two different strategies. First, we labeled a subpopulation of cortical neurons-mainly fast-spiking interneurons-by using adeno-associated virus (AAV) to deliver ChR2 in a transgenic mouse line in which the expression of Cre recombinase was driven by the parvalbumin promoter. Second, we labeled subpopulations of excitatory neurons in the rat auditory cortex with ChR2 based on projection target by using herpes simplex virus 1 (HSV1), which is efficiently taken up by axons and transported retrogradely; we find that this latter population responds to acoustic stimulation differently from unlabeled neurons. Tagging neurons is a novel application of ChR2, used in this case to monitor activity instead of manipulating it. PINP can be readily extended to other populations of genetically identifiable neurons, and will provide a useful method for probing the functional role of different neuronal populations in vivo.
Conflict of interest statement
Figures


Similar articles
-
A guide to in vivo single-unit recording from optogenetically identified cortical inhibitory interneurons.J Vis Exp. 2014 Nov 7;(93):e51757. doi: 10.3791/51757. J Vis Exp. 2014. PMID: 25407742 Free PMC article.
-
Combining Optogenetics and Electrophysiology to Analyze Projection Neuron Circuits.Cold Spring Harb Protoc. 2016 Oct 3;2016(10):pdb.prot090084. doi: 10.1101/pdb.prot090084. Cold Spring Harb Protoc. 2016. PMID: 27698240 Free PMC article.
-
Assessment of the AAV-mediated expression of channelrhodopsin-2 and halorhodopsin in brainstem neurons mediating auditory signaling.Brain Res. 2013 May 20;1511:138-52. doi: 10.1016/j.brainres.2012.10.030. Epub 2012 Oct 23. Brain Res. 2013. PMID: 23088961 Free PMC article. Review.
-
Cell type-specific and time-dependent light exposure contribute to silencing in neurons expressing Channelrhodopsin-2.Elife. 2014;3:e01481. doi: 10.7554/eLife.01481. Epub 2014 Jan 28. Elife. 2014. PMID: 24473077 Free PMC article.
-
Opto-juxtacellular interrogation of neural circuits in freely moving mice.Nat Protoc. 2023 Aug;18(8):2415-2440. doi: 10.1038/s41596-023-00842-7. Epub 2023 Jul 7. Nat Protoc. 2023. PMID: 37420087 Review.
Cited by
-
Arcuate hypothalamic AgRP and putative POMC neurons show opposite changes in spiking across multiple timescales.Elife. 2015 Jul 10;4:e07122. doi: 10.7554/eLife.07122. Elife. 2015. PMID: 26159614 Free PMC article.
-
Serotonergic neurons signal reward and punishment on multiple timescales.Elife. 2015 Feb 25;4:e06346. doi: 10.7554/eLife.06346. Elife. 2015. PMID: 25714923 Free PMC article.
-
Optogenetics in neuroscience: what we gain from studies in mammals.Neurosci Bull. 2012 Aug;28(4):423-34. doi: 10.1007/s12264-012-1250-6. Neurosci Bull. 2012. PMID: 22833040 Free PMC article. Review.
-
Electrophysiological Profiling of Neocortical Neural Subtypes: A Semi-Supervised Method Applied to in vivo Whole-Cell Patch-Clamp Data.Front Neurosci. 2018 Nov 13;12:823. doi: 10.3389/fnins.2018.00823. eCollection 2018. Front Neurosci. 2018. PMID: 30542256 Free PMC article.
-
The functional properties of barrel cortex neurons projecting to the primary motor cortex.J Neurosci. 2010 Mar 24;30(12):4256-60. doi: 10.1523/JNEUROSCI.3774-09.2010. J Neurosci. 2010. PMID: 20335461 Free PMC article.
References
-
- Mountcastle VB, Talbot WH, Sakata H, Hyvarinen J. Cortical neuronal mechanisms in flutter-vibration studied in unanesthetized monkeys. Neuronal periodicity and frequency discrimination. J Neurophysiol. 1969;32:452–484. - PubMed
-
- Steriade M, Timofeev I, Durmuller N, Grenier F. Dynamic properties of corticothalamic neurons and local cortical interneurons generating fast rhythmic (30–40 Hz) spike bursts. J Neurophysiol. 1998;79:483–490. - PubMed
-
- Klausberger T, Magill PJ, Marton LF, Roberts JD, Cobden PM, et al. Brain-state- and cell-type-specific firing of hippocampal interneurons in vivo. Nature. 2003;421:844–848. - PubMed
-
- Nowak LG, Azouz R, Sanchez-Vives MV, Gray CM, McCormick DA. Electrophysiological classes of cat primary visual cortical neurons in vivo as revealed by quantitative analyses. J Neurophysiol. 2003;89:1541–1566. - PubMed
-
- Mitchell JF, Sundberg KA, Reynolds JH. Differential attention-dependent response modulation across cell classes in macaque visual area V4. Neuron. 2007;55:131–141. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

