The Saudi Project for Assessment of Coronary Events (SPACE) registry: design and results of a phase I pilot study
- PMID: 19584982
- PMCID: PMC2723036
- DOI: 10.1016/s0828-282x(09)70513-6
The Saudi Project for Assessment of Coronary Events (SPACE) registry: design and results of a phase I pilot study
Abstract
Objective: The delay between the availability of clinical evidence and its application to the care of patients with acute coronary syndrome (ACS) in the Kingdom of Saudi Arabia remains undefined. The Saudi Project for Assessment of Coronary Events (SPACE) registry provides a comprehensive view of the current diagnostic and treatment strategies for patients with ACS; thus, the registry may be used to identify opportunities to improve the care of these patients.
Methods: Eight hospitals in different regions of Saudi Arabia were involved in the pilot phase of the registry, from December 2005 to July 2006. The study patients included individuals with ST segment elevation myocardial infarction (STEMI), non-STEMI and unstable angina.
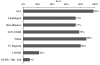
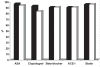
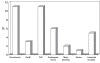
Results: A total of 435 patients (77% men and 80% Saudis) with a mean age of 57.1 years were enrolled. Medical history included previously diagnosed ischemic heart disease (32%), percutaneous coronary intervention (12%), diabetes mellitus (53%), hypertension (48%), current smoking (39%), hyperlipidemia (31%) and family history of premature coronary artery disease (11%). The median door-to-needle time for fibrinolytic therapy received by patients with STEMIs was 90 min. Inhospital medications included acetylsalicylic acid (98%), clopidogrel (73%), angiotensin- converting enzyme inhibitors (74%), beta-blockers (73%), statins (88%), unfractionated heparin (80%), low-molecular weight heparin (22%) and glycoprotein IIb/IIIa inhibitors (9%). The inhospital mortality rate was 5%.
Conclusion: The first nationwide registry of patients with ACS in the Kingdom of Saudi Arabia is presented. In contrast to registries from developed countries, our cohort is characterized by a younger age at presentation and a much higher prevalence of diabetes mellitus. Most patients with STEMIs did not receive fibrinolytic therapy within the time recommended in the American College of Cardiology/American Heart Association guidelines. The results of the present pilot study show potential targets for improvement in care.
OBJECTIF :: On ne connaît pas le délai entre l’obtention des données cliniques et leur application aux soins des patients atteints d’un syndrome coronarien aigu (SCA) dans le Royaume d’Arabie saoudite. Le registre SPACE du projet saoudien pour évaluer les événements coronariens fournit un aperçu complet des stratégies diagnostiques et de traitement des patients ayant un SCA. Ainsi, il peut être utilisé pour repérer des occasions d’améliorer les soins de ces patients.
MÉTHODOLOGIE :: Huit hôpitaux de diverses régions d’Arabie saoudite ont participé à la phase pilote du registre entre décembre 2005 et juillet 2006. Les patients à l’étude incluaient des personnes ayant subi un infarctus du myocarde avec élévation du segment ST (IMÉST), un infarctus du myocarde sans élévation du segment ST (non IMÉST) ou ayant une angine instable.
RÉSULTATS :: Au total, 435 patients (77 % d’hommes et 80 % de Saoudiens), d’un âge moyen de 57,1 ans, ont participé. Leurs antécédents médicaux incluaient une maladie cardiaque ischémique déjà diagnostiquée (32 %), une intervention coronaire percutanée (12 %), le diabète (53 %), l’hypertension (48 %), le tabagisme courant (39 %), l’hyperlipidémie (31 %) et des antécédents familiaux de coronaropathie prématurée (11 %). Le temps médian entre l’arrivée et l’injection pour la thérapie fibrinolytique chez les patients ayant un IMÉST était de 90 minutes. Les médicaments administrés en milieu hospitalier incluaient l’acide acétylsalicylique (98 %), le clopidogrel (73 %), les inhibiteurs de l’enzyme de conversion de l’angiotensine (74 %), les bêta-bloquants (73 %), les statines (88 %), l’héparine non fractionnée (80 %), l’héparine à faible poids moléculaire (22 %) et les inhibiteurs de glycoprotéine IIb/IIIa. Le taux de mortalité en milieu hospitalier était de 5 %.
CONCLUSION :: Le premier registre national de patients ayant un SCA dans le Royaume d’Arabie saoudite est présenté. Contrairement aux registres des pays industrialisés, notre cohorte se caractérise par un plus jeune âge à la présentation et une prévalence beaucoup plus élevée de diabète. La plupart des patients ayant un IMÉST n’avaient pas reçu de thérapie aux fibrinolytiques dans les délais recommandés dans les lignes directrices de l’American College of Cardiology et l’American Heart Association. Les résultats de la présente étude pilote démontrent des cibles potentielles d’amélioration des soins.
Figures
References
-
- WHO . The World Health Report 1998. Geneva: World Health Organization; 1998. pp. 148–85.
-
- Lopez AD, Murray CC. The global burden of disease, 1990–2020. Nat Med. 1998;4:1241–3. - PubMed
-
- Ezzati M, Lopez AD, Rodgers A, Vander Hoorn S, Murray CJ. Selected major risk factors and global and regional burden of disease. Lancet. 2002;360:1347–60. - PubMed
-
- Reddy KS, Yusuf S. Emerging epidemic of cardiovascular disease in developing countries. Circulation. 1998;97:596–601. - PubMed
-
- European Secondary Prevention Study Group Translation of clinical trials into practice: A European population-based study of the use of thrombolysis for acute myocardial infarction. Lancet. 1996;347:1203–7. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

