Cytokeratin-18 fragment levels as noninvasive biomarkers for nonalcoholic steatohepatitis: a multicenter validation study
- PMID: 19585618
- PMCID: PMC2757511
- DOI: 10.1002/hep.23050
Cytokeratin-18 fragment levels as noninvasive biomarkers for nonalcoholic steatohepatitis: a multicenter validation study
Abstract
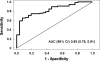
Liver biopsy remains the gold standard for diagnosing nonalcoholic steatohepatitis (NASH). We have recently demonstrated that plasma cytokeratin 18 (CK-18) fragment levels correlate with the magnitude of hepatocyte apoptosis and independently predict the presence of NASH. The goal of this study was to validate the use of this biomarker for NASH diagnosis. The study was an ancillary study of the NASH Clinical Research Network (NASH CRN). Our cohort consisted of 139 patients with biopsy-proven nonalcoholic fatty liver disease (NAFLD) from eight CRN participant centers across the United States and 150 age-matched healthy controls. CK-18 fragments were measured using a specific enzyme-linked immunosorbent assay. Histology was assessed centrally by study pathologists. CK-18 fragments were markedly increased in patients with NASH versus those without NASH and borderline diagnosis (median [25th, 75th percentile], 335 [196, 511], 194 [151, 270], 200 [148, 284], respectively; P < 0.001). Moreover, the odds of having fibrosis on liver biopsy increased with increasing plasma CK-18 fragment levels (P < 0.001). On multivariate regression analysis, CK-18 fragments remained an independent predictor of NASH after adjusting for variables associated with CK-18 fragments or NASH on univariate analysis (fibrosis, alanine aminotransferase, aspartate aminotransferase, age, biopsy length). The area under the receiver operating characteristic curve for NASH diagnosis was estimated to be 0.83 (0.75, 0.91).
Conclusion: Determination of CK-18 fragments in the blood predicts histological NASH and severity of disease in a large, diverse population of patients with biopsy-proven NAFLD, supporting the potential usefulness of this test in clinical practice.
Figures


Comment in
-
One step at a time: identification and validation of biomarkers for nonalcoholic steatohepatitis.Hepatology. 2009 Oct;50(4):1000-3. doi: 10.1002/hep.23288. Hepatology. 2009. PMID: 19787817 No abstract available.
-
Serum markers of hepatocyte apoptosis: current terminology and predictability in clinical practice.Hepatology. 2010 Feb;51(2):717-8. doi: 10.1002/hep.23251. Hepatology. 2010. PMID: 19918977 No abstract available.
-
Serum cytokeratin-18 fragment level: a noninvasive biomarker for not only nonalcoholic steatohepatitis, but also alcoholic steatohepatitis.Hepatology. 2010 May;51(5):1865-6. doi: 10.1002/hep.23433. Hepatology. 2010. PMID: 20041411 No abstract available.
References
-
- Angulo P. Nonalcoholic fatty liver disease. N Engl J Med. 2002;346:1221–1231. - PubMed
-
- Wieckowska A, Feldstein AE. Nonalcoholic fatty liver disease in the pediatric population: a review. Curr Opin Pediatr. 2005;17:636–641. - PubMed
-
- Brunt EM, Janney CG, Di Bisceglie AM, Neuschwander-Tetri BA, Bacon BR. Nonalcoholic steatohepatitis: a proposal for grading and staging the histological lesions. Am J Gastroenterol. 1999;94:2467–2474. - PubMed
-
- Adams LA, Lymp JF, St Sauver J, Sanderson SO, Lindor KD, Feldstein A, Angulo P. The natural history of nonalcoholic fatty liver disease: a population-based cohort study. Gastroenterology. 2005;129:113–121. - PubMed
-
- Ekstedt M, Franzen LE, Mathiesen UL, Thorelius L, Holmqvist M, Bodemar G, Kechagias S. Long-term follow-up of patients with NAFLD and elevated liver enzymes. Hepatology. 2006;44:865–873. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous
