Features of intervertebral disc degeneration in rat's aging process
- PMID: 19585670
- PMCID: PMC2704970
- DOI: 10.1631/jzus.B0820295
Features of intervertebral disc degeneration in rat's aging process
Abstract
Objective: The age-related change is important part of degenerative disc disease. However, no appropriate animal model or objective evaluation index is available. This study aimed to investigate the features of intervertebral disc degeneration in aging process of rats.
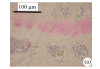
Methods: 22-month-old Sprague-Dawley (SD) rats were used as spontaneously occurring intervertebral disc degeneration models and 6-month-old rats as young controls. Expression of collagen types II and X was measured by immunohistochemistry. Degenerations of intervertebral discs were scored according to Miyamoto's method. Numbers and areas of afferent vascular buds were measured. The thicknesses of non-calcified and calcified layers were measured and statistically analyzed.
Results: There were less collagen type II expression and more collagen type X expression in the calcified layer of the cartilage endplates and nucleus pulposus in the rats of the aged group than in the young control. There were fewer and smaller afferent vascular buds in the rats of the aged group than in the young control group. The ratio of the non-calcified to the calcified layers in the rats of the aged group significantly decreased, compared with that of the young control group (P<0.01).
Conclusion: Rats can spontaneously establish intervertebral disc age-related degeneration. The expression of collagen types II and X, numbers and areas of afferent vascular buds, the ratio of the non-calcified to the calcified layers, and water and glycosaminoglycan contents in the nucleus pulposus are sensitive indexes of intervertebral disc degeneration.
Figures











References
-
- Antoniou J, Steffen T, Nelson F, Winterbottom N, Hollander AP, Poole RA, Aebi M, Alini M. The human lumbar intervertebral disc: evidence for changes in the biosynthesis and denaturation of the extracellular matrix with growth, maturation, ageing, and degeneration. Journal of Clinical Investigation. 1996;98(4):996–1003. doi: 10.1172/JCI118884. - DOI - PMC - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

