Source monitoring 15 years later: what have we learned from fMRI about the neural mechanisms of source memory?
- PMID: 19586165
- PMCID: PMC2859897
- DOI: 10.1037/a0015849
Source monitoring 15 years later: what have we learned from fMRI about the neural mechanisms of source memory?
Abstract
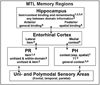
Focusing primarily on functional magnetic resonance imaging (fMRI), this article reviews evidence regarding the roles of subregions of the medial temporal lobes, prefrontal cortex, posterior representational areas, and parietal cortex in source memory. In addition to evidence from standard episodic memory tasks assessing accuracy for neutral information, the article considers studies assessing the qualitative characteristics of memories, the encoding and remembering of emotional information, and false memories, as well as evidence from populations that show disrupted source memory (older adults, individuals with depression, posttraumatic stress disorder, or schizophrenia). Although there is still substantial work to be done, fMRI is advancing understanding of source memory and highlighting unresolved issues. A continued 2-way interaction between cognitive theory, as illustrated by the source monitoring framework (M. K. Johnson, S. Hashtroudi, & D. S. Lindsay, 1993), and evidence from cognitive neuroimaging studies should clarify conceptualization of cognitive processes (e.g., feature binding, retrieval, monitoring), prior knowledge (e.g., semantics, schemas), and specific features (e.g., perceptual and emotional information) and of how they combine to create true and false memories.
Copyright (c) 2009 APA, all rights reserved.
Figures


Comment on
-
Source monitoring.Psychol Bull. 1993 Jul;114(1):3-28. doi: 10.1037/0033-2909.114.1.3. Psychol Bull. 1993. PMID: 8346328 Review.
References
-
- Achim AM, Weiss AP. No evidence for a differential deficit of reality monitoring in schizophrenia: A meta-analysis of the associative memory literature. Cognitive Neuropsychiatry. 2008;13:369–384. - PubMed
-
- Ackil JK, Zaragoza MS. Memorial consequences of forced confabulation: Age differences in susceptibility to false memories. Developmental Psychology. 1998;34:1358–1372. - PubMed
-
- Addis DR, Moscovitch M, Crawley AP, McAndrews MP. Recollective qualities modulate hippocampal activation during autobiographical memory retrieval. Hippocampus. 2004;14:752–762. - PubMed
-
- Aggleton JP, Brown MW. Episodic memory, amnesia, and the hippocampal-anterior thalamic axis. Behavioral and Brain Sciences. 1999;22:425–489. - PubMed
-
- Aleman A, Hijman R, De Haan EHF, Kahn RS. Memory impairment in schizophrenia: a meta-analysis. American Journal of Psychiatry. 1999;156:1358–1366. - PubMed

