Layer by layer three-dimensional tissue epitaxy by cell-laden hydrogel droplets
- PMID: 19586367
- PMCID: PMC2818246
- DOI: 10.1089/ten.TEC.2009.0179
Layer by layer three-dimensional tissue epitaxy by cell-laden hydrogel droplets
Abstract
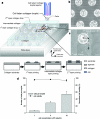
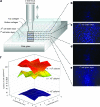
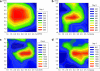
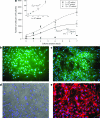
The ability to bioengineer three-dimensional (3D) tissues is a potentially powerful approach to treat diverse diseases such as cancer, loss of tissue function, or organ failure. Traditional tissue engineering methods, however, face challenges in fabricating 3D tissue constructs that resemble the native tissue microvasculature and microarchitectures. We have developed a bioprinter that can be used to print 3D patches of smooth muscle cells (5 mm x 5 mm x 81 microm) encapsulated within collagen. Current inkjet printing systems suffer from loss of cell viability and clogging. To overcome these limitations, we developed a system that uses mechanical valves to print high viscosity hydrogel precursors containing cells. The bioprinting platform that we developed enables (i) printing of multilayered 3D cell-laden hydrogel structures (16.2 microm thick per layer) with controlled spatial resolution (proximal axis: 18.0 +/- 7.0 microm and distal axis: 0.5 +/- 4.9 microm), (ii) high-throughput droplet generation (1 s per layer, 160 droplets/s), (iii) cell seeding uniformity (26 +/- 2 cells/mm(2) at 1 million cells/mL, 122 +/- 20 cells/mm(2) at 5 million cells/mL, and 216 +/- 38 cells/mm(2) at 10 million cells/mL), and (iv) long-term viability in culture (>90%, 14 days). This platform to print 3D tissue constructs may be beneficial for regenerative medicine applications by enabling the fabrication of printed replacement tissues.
Figures



References
-
- Langer R. Vacanti J.P. Tissue engineering. Science. 1993;260:920. - PubMed
-
- Atala A. Bauer S.B. Soker S. Yoo J.J. Retik A.B. Tissue-engineered autologous bladders for patients needing cystoplasty. Lancet. 2006;367:1241. - PubMed
-
- Macchiarini P. Jungebluth P. Go T. Asnaghi M.A. Rees L.E. Cogan T.A. Dodson A. Martorell J. Bellini S. Parnigotto P.P. Dickinson S.C. Hollander A.P. Mantero S. Conconi M.T. Birchall M.A. Clinical transplantation of a tissue-engineered airway. Lancet. 2008;372:2023. - PubMed
-
- Nerem R.M. Cellular engineering. Ann Biomed Eng. 1991;19:529. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

