Inflamed lymphatic endothelium suppresses dendritic cell maturation and function via Mac-1/ICAM-1-dependent mechanism
- PMID: 19587009
- PMCID: PMC4410990
- DOI: 10.4049/jimmunol.0802167
Inflamed lymphatic endothelium suppresses dendritic cell maturation and function via Mac-1/ICAM-1-dependent mechanism
Abstract
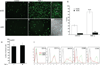
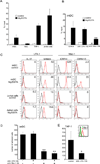
The lymphatic system is essential for the generation of immune responses by facilitating immune cell trafficking to lymph nodes. Dendritic cells (DCs), the most potent APCs, exit tissues via lymphatic vessels, but the mechanisms of interaction between DCs and the lymphatic endothelium and the potential implications of these interactions for immune responses are poorly understood. In this study, we demonstrate that lymphatic endothelial cells (LECs) modulate the maturation and function of DCs. Direct contact of human monocyte-derived DCs with an inflamed, TNF-alpha-stimulated lymphatic endothelium reduced expression of the costimulatory molecule CD86 by DCs and suppressed the ability of DCs to induce T cell proliferation. These effects were dependent on adhesive interactions between DCs and LECs that were mediated by the binding of Mac-1 on DCs to ICAM-1 on LECs. Importantly, the suppressive effects of the lymphatic endothelium on DCs were observed only in the absence of pathogen-derived signals. In vivo, DCs that migrated to the draining lymph nodes upon inflammatory stimuli, but in the absence of a pathogen, showed increased levels of CD86 expression in ICAM-1-deficient mice. Together, these data demonstrate a direct role of LECs in the modulation of immune response and suggest a function of the lymphatic endothelium in preventing undesired immune reactions in inflammatory conditions.
Figures






References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

