Role for the paramyxovirus genomic promoter in limiting host cell antiviral responses and cell killing
- PMID: 19587032
- PMCID: PMC2738250
- DOI: 10.1128/JVI.01055-09
Role for the paramyxovirus genomic promoter in limiting host cell antiviral responses and cell killing
Abstract
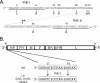
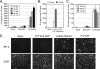
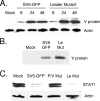
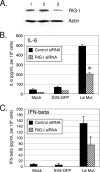
The parainfluenza virus simian virus 5 (SV5) is a poor inducer of innate immune responses. In contrast, the naturally occurring SV5 variant Wake Forest parainfluenza virus (WF-PIV) activates the synthesis of proinflammatory cytokines and beta interferon (IFN-beta). Comparison of SV5 and WF-PIV genome sequences revealed nine nucleotide differences within the viral genomic promoter, including two substitutions (U5C and A14G) in the most highly conserved 3'-end promoter element. To test the consequences of these promoter variations, a recombinant SV5 mutant [Le-(U5C, A14G)] was engineered to harbor the two WF-PIV genomic promoter substitutions in an otherwise wild-type (WT) SV5 background. Human lung epithelial cells infected with the Le-(U5C, A14G) mutant had higher rates of viral protein synthesis and levels of mRNA than cells infected with WT SV5, but levels of genomic RNA were not changed. Unlike WT SV5, the Le-(U5C, A14G) mutant was a potent inducer of interleukin-6 and IFN-beta synthesis, despite expressing a functional V protein antagonist. Cytokine responses to Le-(U5C, A14G) infection were reduced either by small interfering RNA-mediated knockdown of retinoic acid-inducible gene I (RIG-I) or after infection of cells that were engineered to express the reovirus sigma3 double-stranded RNA-binding protein. Le-(U5C, A14G) induced cytopathic effects not seen with WT SV5, and the extent of cell killing correlated with elevated levels of viral F protein and cell-cell fusion. Our results support a model whereby the SV5 promoter has evolved to function at an attenuated level in order to limit (i) synthesis of aberrant RNAs which induce RIG-I-mediated responses and (ii) overproduction of mRNA for potentially toxic gene products, such as the F protein. Control of genomic promoter activity may be particularly important for viruses such as SV5, that express a V protein targeting mda-5 but do not encode antagonists such as the paramyxovirus C proteins, that specifically target RIG-I.
Figures




Similar articles
-
A simian virus 5 (SV5) P/V mutant is less cytopathic than wild-type SV5 in human dendritic cells and is a more effective activator of dendritic cell maturation and function.J Virol. 2006 Apr;80(7):3416-27. doi: 10.1128/JVI.80.7.3416-3427.2006. J Virol. 2006. PMID: 16537609 Free PMC article.
-
TLR3-dependent upregulation of RIG-I leads to enhanced cytokine production from cells infected with the parainfluenza virus SV5.Virology. 2010 Feb 5;397(1):231-41. doi: 10.1016/j.virol.2009.11.014. Epub 2009 Nov 30. Virology. 2010. PMID: 19948350 Free PMC article.
-
Variants of the paramyxovirus Simian virus 5 with accelerated or delayed viral gene expression activate proinflammatory cytokine synthesis.Virology. 2006 Jun 20;350(1):90-102. doi: 10.1016/j.virol.2006.01.006. Epub 2006 Feb 15. Virology. 2006. PMID: 16480754
-
In vitro production of synthetic viral RNAs and their delivery into mammalian cells and the application of viral RNAs in the study of innate interferon responses.Methods. 2020 Nov 1;183:21-29. doi: 10.1016/j.ymeth.2019.10.013. Epub 2019 Nov 1. Methods. 2020. PMID: 31682923 Review.
-
Recognition of Reovirus RNAs by the Innate Immune System.Viruses. 2020 Jun 20;12(6):667. doi: 10.3390/v12060667. Viruses. 2020. PMID: 32575691 Free PMC article. Review.
Cited by
-
Activation of human macrophages by bacterial components relieves the restriction on replication of an interferon-inducing parainfluenza virus 5 (PIV5) P/V mutant.Microbes Infect. 2011 Apr;13(4):359-68. doi: 10.1016/j.micinf.2010.12.005. Epub 2010 Dec 24. Microbes Infect. 2011. PMID: 21185944 Free PMC article.
-
Parainfluenza virus 5-based vaccine vectors expressing vaccinia virus (VACV) antigens provide long-term protection in mice from lethal intranasal VACV challenge.Virology. 2011 Oct 25;419(2):97-106. doi: 10.1016/j.virol.2011.08.005. Epub 2011 Aug 31. Virology. 2011. PMID: 21885079 Free PMC article.
-
RIG-I-like receptors and negative-strand RNA viruses: RLRly bird catches some worms.Cytokine Growth Factor Rev. 2014 Oct;25(5):621-8. doi: 10.1016/j.cytogfr.2014.05.004. Epub 2014 May 20. Cytokine Growth Factor Rev. 2014. PMID: 24894317 Free PMC article. Review.
-
Parainfluenza virus 5 upregulates CD55 expression to produce virions with enhanced resistance to complement-mediated neutralization.Virology. 2016 Oct;497:305-313. doi: 10.1016/j.virol.2016.07.030. Epub 2016 Aug 6. Virology. 2016. PMID: 27505156 Free PMC article.
-
Mumps virus inhibits migration of primary human macrophages toward a chemokine gradient through a TNF-alpha dependent mechanism.Virology. 2012 Nov 10;433(1):245-52. doi: 10.1016/j.virol.2012.08.017. Epub 2012 Aug 28. Virology. 2012. PMID: 22935226 Free PMC article.
References
-
- Biron, C. A., and G. C. Sen. 2007. Innate responses to viral infections, p. 249-278. In D. M. Knipe, P. M. Howley, D. E. Griffin, R. A. Lamb, M. A. Martin, B. Roizman, and S. E. Straus (ed.), Fields virology, 5th ed. Lippincott Williams & Wilkins, Philadelphia, PA.
-
- Childs, K. S., N. Stock, C. Ross, J. Andrejeva, L. Hilton, M. Skinner, R. Randall, and S. Goodbourn. 2007. mda-5, but not RIG-I, is a common target for paramyxovirus V proteins. Virology 359:190-200. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

