Structural and enzymatic characterization of Os3BGlu6, a rice beta-glucosidase hydrolyzing hydrophobic glycosides and (1->3)- and (1->2)-linked disaccharides
- PMID: 19587102
- PMCID: PMC2735989
- DOI: 10.1104/pp.109.139436
Structural and enzymatic characterization of Os3BGlu6, a rice beta-glucosidase hydrolyzing hydrophobic glycosides and (1->3)- and (1->2)-linked disaccharides
Abstract
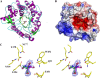
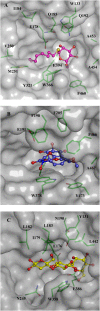
Glycoside hydrolase family 1 (GH1) beta-glucosidases play roles in many processes in plants, such as chemical defense, alkaloid metabolism, hydrolysis of cell wall-derived oligosaccharides, phytohormone regulation, and lignification. However, the functions of most of the 34 GH1 gene products in rice (Oryza sativa) are unknown. Os3BGlu6, a rice beta-glucosidase representing a previously uncharacterized phylogenetic cluster of GH1, was produced in recombinant Escherichia coli. Os3BGlu6 hydrolyzed p-nitrophenyl (pNP)-beta-d-fucoside (k(cat)/K(m) = 67 mm(-1) s(-1)), pNP-beta-d-glucoside (k(cat)/K(m) = 6.2 mm(-1) s(-1)), and pNP-beta-d-galactoside (k(cat)/K(m) = 1.6 mm(-1)s(-1)) efficiently but had little activity toward other pNP glycosides. It also had high activity toward n-octyl-beta-d-glucoside and beta-(1-->3)- and beta-(1-->2)-linked disaccharides and was able to hydrolyze apigenin beta-glucoside and several other natural glycosides. Crystal structures of Os3BGlu6 and its complexes with a covalent intermediate, 2-deoxy-2-fluoroglucoside, and a nonhydrolyzable substrate analog, n-octyl-beta-d-thioglucopyranoside, were solved at 1.83, 1.81, and 1.80 A resolution, respectively. The position of the covalently trapped 2-F-glucosyl residue in the enzyme was similar to that in a 2-F-glucosyl intermediate complex of Os3BGlu7 (rice BGlu1). The side chain of methionine-251 in the mouth of the active site appeared to block the binding of extended beta-(1-->4)-linked oligosaccharides and interact with the hydrophobic aglycone of n-octyl-beta-d-thioglucopyranoside. This correlates with the preference of Os3BGlu6 for short oligosaccharides and hydrophobic glycosides.
Figures

References
-
- Babcock GD, Esen A (1994) Substrate specificity of maize β-glucosidase. Plant Sci 101 31–39
-
- Bachem CW, van der Höven RS, De Bruijin SM, Vreugdenhil D, Zabeau M, Visser RG (1996) Visualization of differential gene expression using novel method of RNA fingerprinting based on AFLP: analysis of gene expression during potato tuber development. Plant J 9 745–753 - PubMed
-
- Barrett T, Suresh CG, Tolley SP, Dodson EJ, Hughes MA (1995) The crystal structure of a cyanogenic β-glucosidase from white clover, a family 1 glycosyl hydrolase. Structure 3 951–960 - PubMed
-
- Bendtsen JD, Nielsen H, von Heijne G, Brunak S (2004) Improved prediction of signal peptides: SignalP 3.0. J Mol Biol 340 783–795 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

