Characterization of the kinase activity of a WNK4 protein complex
- PMID: 19587141
- PMCID: PMC2739714
- DOI: 10.1152/ajprenal.00358.2009
Characterization of the kinase activity of a WNK4 protein complex
Abstract
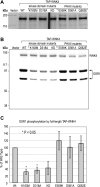
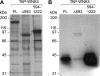
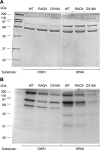
Mutations in WNK4 protein kinase cause pseudohypoaldosteronism type II (PHAII), a genetic disorder that is characterized by renal NaCl and K(+) retention leading to hypertension and hyperkalemia. Consistent with this, WNK4 is known to regulate several renal tubule transporters, including the NaCl cotransporter, NCC, and the K(+) channel, ROMK, but the mechanisms are incompletely understood, and the role of the kinase activity in its actions is highly controversial. To assay WNK4 kinase activity, we have now succeeded in expressing and purifying full-length, enzymatically active WNK4 protein from HEK293 cells. We show that full-length wild-type WNK4 phosphorylates oxidative stress response kinase 1 (OSR1) and Ste20/SPS1-related proline/alanine-rich kinase (SPAK) in vitro. Introducing the PHAII-associated mutations, E559K, D561A, and Q562E, into our protein had no significant effect on this phosphorylation. We conclude that PHAII is unlikely to be caused by abnormal WNK4 kinase activity. We also made the intriguing observation that inactivating mutations of the WNK4 kinase domain did not completely abolish in vitro phosphorylation of OSR1/SPAK. Led by this, we identified a novel 40-kDa kinase that associates specifically with the COOH-terminal half of WNK4 and is able to phosphorylate both WNK4 and SPAK/OSR1. We suggest that this 40-kDa kinase functions in the WNK4 signal transduction pathway and may mediate some of the physiological actions attributed to WNK4.
Figures






References
-
- Gagnon KB, England R, Delpire E. Volume sensitivity of cation-Cl− cotransporters is modulated by the interaction of two kinases: Ste20-related proline-alanine-rich kinase and WNK4. Am J Physiol Cell Physiol 290: C134–C142, 2006. - PubMed
-
- Kahle KT, Wilson FH, Leng Q, Lalioti MD, O'Connell AD, Dong K, Rapson AK, MacGregor GG, Giebisch G, Hebert SC, Lifton RP. WNK4 regulates the balance between renal NaCl reabsorption and K+ secretion. Nat Genet 35: 372–376, 2003. - PubMed
-
- Lenertz LY, Lee BH, Min X, Xu BE, Wedin K, Earnest S, Goldsmith EJ, Cobb MH. Properties of WNK1 and implications for other family members. J Biol Chem 280: 26653–26658, 2005. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

