GEC-targeted HO-1 expression reduces proteinuria in glomerular immune injury
- PMID: 19587144
- PMCID: PMC2739711
- DOI: 10.1152/ajprenal.00213.2009
GEC-targeted HO-1 expression reduces proteinuria in glomerular immune injury
Erratum in
- Am J Physiol Renal Physiol. 2009 Nov;297(5):F1476
Abstract
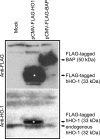
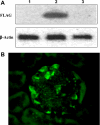
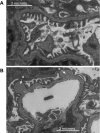
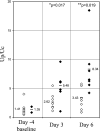
Induction of heme oxygenase (HO)-1 is a key defense mechanism against oxidative stress. Compared with tubules, glomeruli are refractory to HO-1 upregulation in response to injury. This can be a disadvantage as it may be associated with insufficient production of cytoprotective heme-degradation metabolites. We, therefore, explored whether 1) targeted HO-1 expression can be achieved in glomeruli without altering their physiological integrity and 2) this expression reduces proteinuria in immune injury induced by an anti-glomerular basement membrane (GBM) antibody (Ab). We employed a 4.125-kb fragment of a mouse nephrin promoter downstream to which a FLAG-tagged hHO-1 cDNA sequence was inserted and subsequently generated transgenic mice from the FVB/N parental strain. There was a 16-fold higher transgene expression in the kidney than nonspecific background (liver) while the transprotein immunolocalized in glomerular epithelial cells (GEC). There was no change in urinary protein excretion, indicating that GEC-targeted HO-1 expression had no effect on glomerular protein permeability. Urinary protein excretion in transgenic mice with anti-GBM Ab injury (days 3 and 6) was significantly lower compared with wild-type controls. There was no significant change in renal expression levels of profibrotic (TGF-beta1) or anti-inflammatory (IL-10) cytokines in transgenic mice with anti-GBM Ab injury. These observations indicate that GEC-targeted HO-1 expression does not alter glomerular physiological integrity and reduces proteinuria in glomerular immune injury.
Figures







Similar articles
-
Glomerular Epithelial Cells-Targeted Heme Oxygenase-1 Over Expression in the Rat: Attenuation of Proteinuria in Secondary But Not Primary Injury.Nephron. 2016;133(4):270-8. doi: 10.1159/000445755. Epub 2016 Jul 22. Nephron. 2016. PMID: 27442135
-
Heme Oxygenase 1 Up-Regulates Glomerular Decay Accelerating Factor Expression and Minimizes Complement Deposition and Injury.Am J Pathol. 2016 Nov;186(11):2833-2845. doi: 10.1016/j.ajpath.2016.07.009. Epub 2016 Sep 21. Am J Pathol. 2016. PMID: 27662796
-
Mechanisms of HO-1 mediated attenuation of renal immune injury: a gene profiling study.Transl Res. 2011 Oct;158(4):249-61. doi: 10.1016/j.trsl.2011.06.001. Epub 2011 Jul 5. Transl Res. 2011. PMID: 21925121
-
Pathogenesis of glomerular damage in glomerulonephritis.Nephrol Dial Transplant. 1998;13 Suppl 1:10-5. doi: 10.1093/ndt/13.suppl_1.10. Nephrol Dial Transplant. 1998. PMID: 9507491 Review.
-
Pathogenesis of the podocytopathy and proteinuria in diabetic glomerulopathy.Curr Diabetes Rev. 2008 Feb;4(1):39-45. doi: 10.2174/157339908783502370. Curr Diabetes Rev. 2008. PMID: 18220694 Review.
Cited by
-
Bilirubin as a potential causal factor in type 2 diabetes risk: a Mendelian randomization study.Diabetes. 2015 Apr;64(4):1459-69. doi: 10.2337/db14-0228. Epub 2014 Nov 3. Diabetes. 2015. PMID: 25368098 Free PMC article.
-
Total bilirubin is negatively related to diabetes mellitus in Chinese elderly: a community study.Ann Transl Med. 2019 Sep;7(18):474. doi: 10.21037/atm.2019.07.104. Ann Transl Med. 2019. PMID: 31700910 Free PMC article.
-
Metalloporphyrins as Tools for Deciphering the Role of Heme Oxygenase in Renal Immune Injury.Int J Mol Sci. 2023 Apr 6;24(7):6815. doi: 10.3390/ijms24076815. Int J Mol Sci. 2023. PMID: 37047787 Free PMC article. Review.
-
Elevated serum urate is a potential factor in reduction of total bilirubin: a Mendelian randomization study.Oncotarget. 2017 Oct 24;8(61):103864-103873. doi: 10.18632/oncotarget.21977. eCollection 2017 Nov 28. Oncotarget. 2017. PMID: 29262606 Free PMC article.
-
Regulation of Complement Activation by Heme Oxygenase-1 (HO-1) in Kidney Injury.Antioxidants (Basel). 2021 Jan 6;10(1):60. doi: 10.3390/antiox10010060. Antioxidants (Basel). 2021. PMID: 33418934 Free PMC article. Review.
References
-
- Border WA, Noble NA. TGF-beta in kidney fibrosis: a target for gene therapy. Kidney Int 51: 1388–1396, 1997. - PubMed
-
- Bubner B, Baldwin IT. Use of real-time PCR for determining copy number and zygosity in transgenic plants. Plant Cell Rep 23: 263–271, 2004. - PubMed
-
- Datta PK, Duann P, Lianos EA. Long-term effect of heme oxygenase (HO)-1 induction in glomerular immune injury. J Lab Clin Med 147: 150–155, 2006. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

