Putative novel surface-exposed Streptococcus agalactiae protein frequently expressed by the group B streptococcus from Zimbabwe
- PMID: 19587152
- PMCID: PMC2745001
- DOI: 10.1128/CVI.00133-09
Putative novel surface-exposed Streptococcus agalactiae protein frequently expressed by the group B streptococcus from Zimbabwe
Abstract
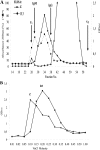
Group B streptococci (GBS) express a variety of surface-exposed and strain-variable proteins which function as phenotypic markers and as antigens which are able to induce protective immunity in experimental settings. Among these proteins, the chimeric and immunologically cross-reacting alpha-like proteins are particularly important. Another protein, R3, which has been less well studied, occurred at a frequency of 21.5% in GBS from Zimbabwe and, notably, occurred in serotype V strains at a frequency of 75.9%. Working with rabbit antiserum raised against the R3 reference strain ATCC 49447 (strain 10/84; serotype V/R3) to detect the expression of the R3 protein, we recorded findings which suggested that strain 10/84 expressed a strain-variable protein antigen, in addition to R3. The antigen was detected by various enzyme-linked immunosorbent assay-based tests by using acid extract antigens or GBS whole-cell coats and by whole-cell-based Western blotting. We named the putative novel antigen the Z antigen. The Z antigen was a high-molecular-mass antigen that was susceptible to degradation by pepsin and trypsin but that was resistant to m-periodate oxidation and failed to show immunological cross-reactivity with any of a variety of other GBS protein antigens. The Z antigen was expressed by 33/121 (27.2%) of strains of a Zimbabwean GBS strain collection and by 64.2% and 72.4% of the type Ib and type V strains, respectively, and was occasionally expressed by GBS of other capsular serotypes. Thus, the putative novel GBS protein named Z showed distinct capsular antigen associations and presented as an important phenotypic marker in GBS from Zimbabwe. It may be an important antigen in GBS from larger areas of southern Africa. Its prevalence in GBS from Western countries is not known.
Figures
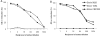


References
-
- Bevanger, L., and J. A. Maeland. 1977. Type classification of group B streptococci by the fluorescent antibody test. Acta Pathol. Microbiol. Immunol. Scand. Sect. B 85:357-362. - PubMed
-
- Bevanger, L., and J. A. Maeland. 1979. Complete and incomplete Ibc protein fraction in group B streptococci. Acta Pathol. Microbiol. Immunol. Scand. Sect. B 87:51-54. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

