Function of beta1 integrin in oral epithelia and tooth bud morphogenesis
- PMID: 19587159
- PMCID: PMC2882240
- DOI: 10.1177/0022034509338008
Function of beta1 integrin in oral epithelia and tooth bud morphogenesis
Abstract
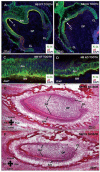
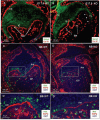
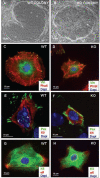
Integrin beta1 is critical for basement membrane organization and hair follicle morphogenesis in the skin epidermis; however, less is known about its function in the developing oral epithelium. Since the skin and oral epithelia share structural similarity, we hypothesized that beta1 integrin function would be critical for the normal development of oral epithelium and tooth buds. The conditional (oral mucosa-specific) beta1 integrin knockout (KO) mice displayed severe disruption of the basement membrane of the tongue epithelium and developing tooth buds. Interestingly, unlike the developing hair follicles, early morphological development of the KO molar tooth buds was normal. However, subsequent morphogenetic events, such as cusp formation, cervical loop down-growth, and ameloblast polarization, did not occur normally. Primary KO oral keratinocytes showed defective cell spreading and robust focal adhesions. Our studies indicate that beta1 integrin plays an essential role in the normal development of the oral epithelium and its appendages.
Figures

References
-
- Ahmed N, Riley C, Rice GE, Quinn MA, Baker MS. (2002). Alpha(v)beta(6) integrin-A marker for the malignant potential of epithelial ovarian cancer. J Histochem Cytochem 50:1371-1380 - PubMed
-
- Arihiro K, Kaneko M, Fujii S, Inai K, Yokosaki Y. (2000). Significance of alpha 9 beta 1 and alpha v beta 6 integrin expression in breast carcinoma. Breast Cancer 7:19-26 - PubMed
-
- Berrier AL, Yamada KM. (2007). Cell-matrix adhesion. J Cell Physiol 213:565-573 - PubMed
-
- Breuss JM, Gallo J, DeLisser HM, Klimanskaya IV, Folkesson HG, Pittet JF, et al. (1995). Expression of the beta 6 integrin subunit in development, neoplasia and tissue repair suggests a role in epithelial remodeling.J Cell Sci 108(Pt 6):2241-2251 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

