Metabolic inhibition increases activity of connexin-32 hemichannels permeable to Ca2+ in transfected HeLa cells
- PMID: 19587218
- PMCID: PMC2740400
- DOI: 10.1152/ajpcell.00200.2009
Metabolic inhibition increases activity of connexin-32 hemichannels permeable to Ca2+ in transfected HeLa cells
Abstract
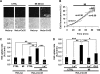
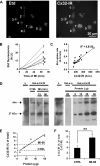
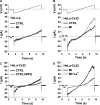
Numerous cell types express functional connexin (Cx) hemichannels (HCs), and membrane depolarization and/or exposure to a divalent cation-free bathing solution (DCFS) have been shown to promote HC opening. However, little is known about conditions that can promote HC opening in the absence of strong depolarization and when extracellular divalent cation concentrations remain at physiological levels. Here the effects of metabolic inhibition (MI), an in vitro model of ischemia, on the activity of mouse Cx32 HCs were examined. In HeLa cells stably transfected with mouse Cx32 (HeLa-Cx32), MI induced an increase in cellular permeability to ethidium (Etd). The increase in Etd uptake was directly related to an increase in levels of Cx32 HCs present at the cell surface. Moreover, MI increased membrane currents in HeLa-Cx32 cells. Underlying these currents were channels exhibiting a unitary conductance of approximately 90 pS, consistent with Cx32 HCs. These currents and Etd uptake were blocked by HC inhibitors. The increase in Cx32 HC activity was preceded by a rapid reduction in mitochondrial membrane potential and a rise in free intracellular Ca(2+) concentration ([Ca(2+)](i)). The increase in free [Ca(2+)](i) was prevented by HC blockade or exposure to extracellular DCFS and was virtually absent in parental HeLa cells. Moreover, inhibition of Cx32 HCs expressed by HeLa cells in low-confluence cultures drastically reduced cell death induced by oxygen-glucose deprivation, which is a more physiological model of ischemia-reperfusion. Thus HC blockade could reduce the increase in free [Ca(2+)](i) and cell death induced by ischemia-like conditions in cells expressing Cx32 HCs.
Figures



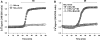

Similar articles
-
On Biophysical Properties and Sensitivity to Gap Junction Blockers of Connexin 39 Hemichannels Expressed in HeLa Cells.Front Physiol. 2017 Feb 9;8:38. doi: 10.3389/fphys.2017.00038. eCollection 2017. Front Physiol. 2017. PMID: 28232803 Free PMC article.
-
A fully human IgG1 antibody targeting connexin 32 extracellular domain blocks CMTX1 hemichannel dysfunction in an in vitro model.Cell Commun Signal. 2024 Dec 5;22(1):589. doi: 10.1186/s12964-024-01969-0. Cell Commun Signal. 2024. PMID: 39639332 Free PMC article.
-
Linoleic acid permeabilizes gastric epithelial cells by increasing connexin 43 levels in the cell membrane via a GPR40- and Akt-dependent mechanism.Biochim Biophys Acta. 2016 May;1861(5):439-48. doi: 10.1016/j.bbalip.2016.02.002. Epub 2016 Feb 8. Biochim Biophys Acta. 2016. PMID: 26869446 Free PMC article.
-
Cell membrane permeabilization via connexin hemichannels in living and dying cells.Exp Cell Res. 2010 Sep 10;316(15):2377-89. doi: 10.1016/j.yexcr.2010.05.026. Epub 2010 Jun 2. Exp Cell Res. 2010. PMID: 20595004 Review.
-
Calcium Regulation of Connexin Hemichannels.Int J Mol Sci. 2024 Jun 15;25(12):6594. doi: 10.3390/ijms25126594. Int J Mol Sci. 2024. PMID: 38928300 Free PMC article. Review.
Cited by
-
On Biophysical Properties and Sensitivity to Gap Junction Blockers of Connexin 39 Hemichannels Expressed in HeLa Cells.Front Physiol. 2017 Feb 9;8:38. doi: 10.3389/fphys.2017.00038. eCollection 2017. Front Physiol. 2017. PMID: 28232803 Free PMC article.
-
Contribution of Astroglial Cx43 Hemichannels to the Modulation of Glutamatergic Currents by D-Serine in the Mouse Prefrontal Cortex.J Neurosci. 2017 Sep 13;37(37):9064-9075. doi: 10.1523/JNEUROSCI.2204-16.2017. Epub 2017 Aug 11. J Neurosci. 2017. PMID: 28821660 Free PMC article.
-
Differentially altered Ca2+ regulation and Ca2+ permeability in Cx26 hemichannels formed by the A40V and G45E mutations that cause keratitis ichthyosis deafness syndrome.J Gen Physiol. 2010 Jul;136(1):47-62. doi: 10.1085/jgp.201010433. J Gen Physiol. 2010. PMID: 20584891 Free PMC article.
-
Atrial fibrillation-associated connexin40 mutants make hemichannels and synergistically form gap junction channels with novel properties.FEBS Lett. 2014 Apr 17;588(8):1458-64. doi: 10.1016/j.febslet.2014.01.010. Epub 2014 Jan 20. FEBS Lett. 2014. PMID: 24457199 Free PMC article.
-
Regulation of hemichannels and gap junction channels by cytokines in antigen-presenting cells.Mediators Inflamm. 2014;2014:742734. doi: 10.1155/2014/742734. Epub 2014 Sep 9. Mediators Inflamm. 2014. PMID: 25301274 Free PMC article. Review.
References
-
- Ankarcrona M, Dypbukt JM, Orrenius S, Nicotera P. Calcineurin and mitochondrial function in glutamate-induced neuronal cell death. FEBS Lett 394: 321–324, 1996 - PubMed
-
- Araya R, Riquelme MA, Brandan E, Sáez JC. The formation of skeletal muscle myotubes requires functional membrane receptors activated by extracellular ATP. Brain Res Rev 47: 174–188, 2004 - PubMed
-
- Bano D, Nicotera P. Ca2+ signals and neuronal death in brain ischemia. Stroke 38: 674–676, 2007 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

