Polysialic acid governs T-cell development by regulating progenitor access to the thymus
- PMID: 19587240
- PMCID: PMC2715481
- DOI: 10.1073/pnas.0905188106
Polysialic acid governs T-cell development by regulating progenitor access to the thymus
Abstract
Although the polysialyltransferase ST8Sia IV is expressed in both primary and secondary human lymphoid organs, its product, polysialic acid (polySia), has been largely overlooked by immunologists. In contrast, polySia expression and function in the nervous system has been well characterized. In this context, polySia modulates cellular adhesion, migration, cytokine response, and contact-dependent differentiation. Provocatively, these same processes are vital components of immune development and function. We previously established that mouse multipotent hematopoietic progenitors use ST8Sia IV to express polySia on their cell surfaces. Here, we demonstrate that, relative to wild-type controls, ST8Sia IV(-/-) mice have a 30% reduction in total thymocytes and a concomitant deficiency in the earliest thymocyte precursors. T-cell progenitors originate in the bone marrow and are mobilized to the blood at regular intervals by unknown signals. We performed in vivo reconstitution experiments in which ST8Sia IV(-/-) progenitors competed with wild-type cells to repopulate depleted or deficient immune subsets. Progenitors lacking polySi exhibited a specific defect in T-cell development because of an inability to access the thymus. This phenotype probably reflects a decreased capacity of the ST8Sia IV(-/-) progenitors to escape from the bone marrow niche. Collectively, these results provide evidence that polySia is involved in hematopoietic development.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



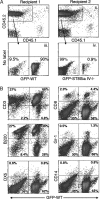
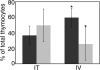
References
-
- Rossi FM, et al. Recruitment of adult thymic progenitors is regulated by P-selectin and its ligand PSGL-1. Nat Immunol. 2005;6(6):626–634. - PubMed
-
- Schwarz BA, Bhandoola A. Circulating hematopoietic progenitors with T lineage potential. Nat Immunol. 2004;5(9):953–960. - PubMed
-
- Kiel MJ, et al. SLAM family receptors distinguish hematopoietic stem and progenitor cells and reveal endothelial niches for stem cells. Cell. 2005;121(7):1109–1121. - PubMed
-
- Kiel MJ, Morrison SJ. Maintaining hematopoietic stem cells in the vascular niche. Immunity. 2006;25(6):862–864. - PubMed
-
- Calvi LM, et al. Osteoblastic cells regulate the haematopoietic stem cell niche. Nature. 2003;425(6960):841–846. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

