Leptin receptor is expressed in thymus medulla and leptin protects against thymic remodeling during endotoxemia-induced thymus involution
- PMID: 19587263
- PMCID: PMC3747557
- DOI: 10.1677/JOE-09-0179
Leptin receptor is expressed in thymus medulla and leptin protects against thymic remodeling during endotoxemia-induced thymus involution
Abstract
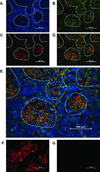
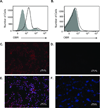
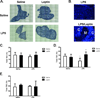
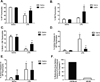
Leptin deficiency in mice results in chronic thymic atrophy, suppressed cell-mediated immunity, and decreased numbers of total lymphocytes, suggesting a key role for the metabolic hormone leptin in regulating thymopoiesis and overall immune homeostasis. Unfortunately, the thymus is highly susceptible to stress-induced acute involution. Prolonged thymus atrophy in stress situations can contribute to peripheral T cell deficiency or inhibit immune reconstitution. Little is known, however, about specific roles for leptin signaling in the thymus or the underlying mechanisms driving thymic involution or thymic recovery after acute stress. We report here that leptin receptor expression is restricted in thymus to medullary epithelial cells. Using a model of endotoxemia-induced acute thymic involution and recovery, we have demonstrated a role for supraphysiologic leptin in protection of thymic epithelial cells (TECs). We also present data in support of our hypothesis that leptin treatment decreases in vivo endotoxemia-induced apoptosis of double positive thymocytes and promotes proliferation of double negative thymocytes through a leptin receptor isoform b-specific mechanism. Furthermore, our studies have revealed that leptin treatment increases thymic expression of interleukin-7, an important soluble thymocyte growth factor produced by medullary TECs. Taken together, these studies support an intrathymic role for the metabolic hormone leptin in maintaining healthy thymic epithelium and promoting thymopoiesis, which is revealed when thymus homeostasis is perturbed by endotoxemia.
Conflict of interest statement
The authors have no financial conflicts of interest.
Figures
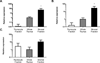

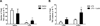
References
-
- Ahima RS, Osei SY. Leptin signaling. Physiology and Behavior. 2004;81:223–241. - PubMed
-
- Bjorbaek C, Uotani S, da Silva B, Flier JS. Divergent signaling capacities of the long and short isoforms of the leptin receptor. Journal of Biological Chemistry. 1997;272:32686–32695. - PubMed
-
- Cohen P, Yang G, Yu X, Soukas AA, Wolfish CS, Friedman JM, Li C. Induction of leptin receptor expression in the liver by leptin and food deprivation. Journal of Biological Chemistry. 2005;280:10034–10039. - PubMed
-
- De Rosa V, Procaccini C, Cali G, Pirozzi G, Fontana S, Zappacosta S, La Cava A, Matarese G. A key role of leptin in the control of regulatory T cell proliferation. Immunity. 2007;26:241–255. - PubMed
-
- Erickson M, Morkowski S, Lehar S, Gillard G, Beers C, Dooley J, Rubin JS, Rudensky A, Farr AG. Regulation of thymic epithelium by keratinocyte growth factor. Blood. 2002;100:3269–3278. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

