Alpha-latrotoxin stimulates a novel pathway of Ca2+-dependent synaptic exocytosis independent of the classical synaptic fusion machinery
- PMID: 19587270
- PMCID: PMC2739239
- DOI: 10.1523/JNEUROSCI.0898-09.2009
Alpha-latrotoxin stimulates a novel pathway of Ca2+-dependent synaptic exocytosis independent of the classical synaptic fusion machinery
Abstract
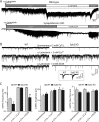
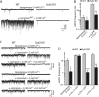
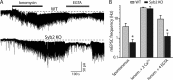
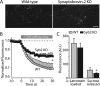
Alpha-latrotoxin induces neurotransmitter release by stimulating synaptic vesicle exocytosis via two mechanisms: (1) A Ca(2+)-dependent mechanism with neurexins as receptors, in which alpha-latrotoxin acts like a Ca(2+) ionophore, and (2) a Ca(2+)-independent mechanism with CIRL/latrophilins as receptors, in which alpha-latrotoxin directly stimulates the transmitter release machinery. Here, we show that the Ca(2+)-independent release mechanism by alpha-latrotoxin requires the synaptic SNARE-proteins synaptobrevin/VAMP and SNAP-25, and, at least partly, the synaptic active-zone protein Munc13-1. In contrast, the Ca(2+)-dependent release mechanism induced by alpha-latrotoxin does not require any of these components of the classical synaptic release machinery. Nevertheless, this type of exocytotic neurotransmitter release appears to fully operate at synapses, and to stimulate exocytosis of the same synaptic vesicles that participate in physiological action potential-triggered release. Thus, synapses contain two parallel and independent pathways of Ca(2+)-triggered exocytosis, a classical, physiological pathway that operates at the active zone, and a novel reserve pathway that is recruited only when Ca(2+) floods the synaptic terminal.
Figures

Similar articles
-
alpha-Latrotoxin and its receptors: neurexins and CIRL/latrophilins.Annu Rev Neurosci. 2001;24:933-62. doi: 10.1146/annurev.neuro.24.1.933. Annu Rev Neurosci. 2001. PMID: 11520923 Review.
-
alpha-Latrotoxin, acting via two Ca2+-dependent pathways, triggers exocytosis of two pools of synaptic vesicles.J Biol Chem. 2001 Nov 30;276(48):44695-703. doi: 10.1074/jbc.M108088200. Epub 2001 Sep 25. J Biol Chem. 2001. PMID: 11572875
-
Mechanisms of alpha-latrotoxin action.Cell Tissue Res. 1999 May;296(2):229-33. doi: 10.1007/s004410051284. Cell Tissue Res. 1999. PMID: 10382267 Review.
-
Alpha-latrotoxin triggers extracellular Ca(2+)-dependent exocytosis and sensitizes fusion machinery in endocrine cells.Acta Biochim Biophys Sin (Shanghai). 2006 Jan;38(1):8-14. doi: 10.1111/j.1745-7270.2006.00129.x. Acta Biochim Biophys Sin (Shanghai). 2006. PMID: 16395521
-
alpha-Latrotoxin stimulates exocytosis by the interaction with a neuronal G-protein-coupled receptor.Neuron. 1997 Jun;18(6):925-37. doi: 10.1016/s0896-6273(00)80332-3. Neuron. 1997. PMID: 9208860
Cited by
-
The role of non-canonical SNAREs in synaptic vesicle recycling.Cell Logist. 2012 Jan 1;2(1):20-27. doi: 10.4161/cl.20114. Cell Logist. 2012. PMID: 22645707 Free PMC article.
-
A Comprehensive Mutagenesis Screen of the Adhesion GPCR Latrophilin-1/ADGRL1.iScience. 2018 May 25;3:264-278. doi: 10.1016/j.isci.2018.04.019. Epub 2018 Apr 30. iScience. 2018. PMID: 30428326 Free PMC article.
-
Vti1a identifies a vesicle pool that preferentially recycles at rest and maintains spontaneous neurotransmission.Neuron. 2012 Jan 12;73(1):121-34. doi: 10.1016/j.neuron.2011.10.034. Neuron. 2012. PMID: 22243751 Free PMC article.
-
The latrophilins, "split-personality" receptors.Adv Exp Med Biol. 2010;706:59-75. doi: 10.1007/978-1-4419-7913-1_5. Adv Exp Med Biol. 2010. PMID: 21618826 Free PMC article. Review.
-
New perspective on sustained antidepressant effect: focus on neurexins regulating synaptic plasticity.Cell Death Discov. 2024 May 1;10(1):205. doi: 10.1038/s41420-024-01974-9. Cell Death Discov. 2024. PMID: 38693106 Free PMC article. Review.
References
-
- Ádám-Vizi V, Déri Z, Bors P, Tretter L. Lack of involvement of [Ca2+]i in the external Ca2+-independent release of acetylcholine evoked by veratridine, ouabain and alpha-latrotoxin: possible role of [Na+]i . J Physiol Paris. 1993;87:43–50. - PubMed
-
- Augustin I, Rosenmund C, Südhof TC, Brose N. Munc13-1 is essential for fusion competence of glutamatergic synaptic vesicles. Nature. 1999;400:457–461. - PubMed
-
- Bronk P, Deák F, Wilson MC, Liu X, Südhof TC, Kavalali ET. Differential effects of SNAP-25 deletion on Ca2+-dependent and Ca2+-independent neurotransmission. J Neurophysiol. 2007;98:794–806. - PubMed
-
- Brose N, Hofmann K, Hata Y, Südhof TC. Mammalian homologues of Caenorhabditis elegans unc-13 gene define novel family of C2-domain proteins. J Biol Chem. 1995;270:25273–25280. - PubMed
-
- Capogna M, Gähwiler BH, Thompson SM. Calcium-independent actions of α-latrotoxin on spontaneous and evoked synaptic transmission in the hippocampus. J Neurophysiol. 1996;76:3149–3158. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous
