Phosphorylation of prion protein at serine 43 induces prion protein conformational change
- PMID: 19587281
- PMCID: PMC2745063
- DOI: 10.1523/JNEUROSCI.2294-09.2009
Phosphorylation of prion protein at serine 43 induces prion protein conformational change
Abstract
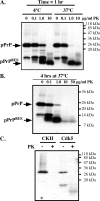
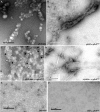
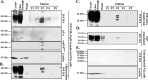
The cause of the conformational change of normal cellular prion protein (PrP) into its disease-associated form is unknown. Posttranslational modifications, such as glycosylation, acetylation, S-nitrosylation, and phosphorylation, are known to induce protein conformational changes. Here, we investigated whether phosphorylation could induce the conformational change of PrP because PrP contains several kinase motifs and has been found recently in the cytosol, in which kinases generally reside. Neuronal cyclin-dependent kinase 5 (Cdk5) phosphorylated recombinant PrP(23-231) at serine 43 (S43) in an in vitro kinase assay. Cdk5-phosphorylated PrP became proteinase K resistant, formed Congo Red-positive fibrils, and formed aggregates that were immunostained with anti-PrP and anti-phospho-PrP(S43) (anti-pPrP(S43)). pPrP(S43) was detected in PrP/Cdk5/p25 cotransfected N2a cells. Roscovitine inhibition of Cdk5 activity or transfection of N2a cells with mutant PrP S43A eliminated the anti-pPrP(S43)-immunopositive protein. Alkaline phosphatase-sensitive and proteinase K-resistant pPrP(S43) immunoreactivity was observed in scrapie-infected but not control-injected mice brains. These results raise the possibility that phosphorylation could represent a physiological mechanism of PrP conversion in vivo.
Figures





Similar articles
-
Cyclin-dependent kinase 5 phosphorylation of familial prion protein mutants exacerbates conversion into amyloid structure.J Biol Chem. 2015 Feb 27;290(9):5759-71. doi: 10.1074/jbc.M114.630699. Epub 2015 Jan 8. J Biol Chem. 2015. PMID: 25572400 Free PMC article.
-
Formation of soluble oligomers and amyloid fibrils with physical properties of the scrapie isoform of the prion protein from the C-terminal domain of recombinant murine prion protein mPrP-(121-231).J Biol Chem. 2006 Sep 8;281(36):26121-8. doi: 10.1074/jbc.M605367200. Epub 2006 Jul 13. J Biol Chem. 2006. PMID: 16844683
-
In vitro evaluation of the anti-prionic activity of newly synthesized congo red derivatives.Arzneimittelforschung. 2003;53(12):875-88. doi: 10.1055/s-0031-1299845. Arzneimittelforschung. 2003. PMID: 14750496
-
Properties and pathogenicity of prion-derived peptides.Protein Pept Lett. 2009;16(3):230-8. doi: 10.2174/092986609787601651. Protein Pept Lett. 2009. PMID: 19275735 Review.
-
Post-translational modifications in prion proteins.Curr Protein Pept Sci. 2002 Dec;3(6):643-52. doi: 10.2174/1389203023380440. Curr Protein Pept Sci. 2002. PMID: 12470218 Review.
Cited by
-
Baking a mass-spectrometry data PIE with McMC and simulated annealing: predicting protein post-translational modifications from integrated top-down and bottom-up data.Bioinformatics. 2011 Mar 15;27(6):844-52. doi: 10.1093/bioinformatics/btr027. Bioinformatics. 2011. PMID: 21389073 Free PMC article.
-
Parkinson's Disease: A Prionopathy?Int J Mol Sci. 2021 Jul 27;22(15):8022. doi: 10.3390/ijms22158022. Int J Mol Sci. 2021. PMID: 34360787 Free PMC article. Review.
-
Effects of peptidyl-prolyl isomerase 1 depletion in animal models of prion diseases.Prion. 2018 Mar 4;12(2):127-137. doi: 10.1080/19336896.2018.1464367. Epub 2018 May 18. Prion. 2018. PMID: 29676205 Free PMC article.
-
The N-terminal sequence of prion protein consists an epitope specific to the abnormal isoform of prion protein (PrP(Sc)).PLoS One. 2013;8(2):e58013. doi: 10.1371/journal.pone.0058013. Epub 2013 Feb 28. PLoS One. 2013. PMID: 23469131 Free PMC article.
-
Big versus small: The impact of aggregate size in disease.Protein Sci. 2023 Jul;32(7):e4686. doi: 10.1002/pro.4686. Protein Sci. 2023. PMID: 37243896 Free PMC article. Review.
References
-
- Aguzzi A, Polymenidou M. Mammalian prion biology: one century of evolving concepts. Cell. 2004;116:313–327. - PubMed
-
- Arai T, Guo JP, McGeer PL. Proteolysis of non-phosphorylated and phosphorylated tau by thrombin. J Biol Chem. 2005;280:5145–5153. - PubMed
-
- Atarashi R, Moore RA, Sim VL, Hughson AG, Dorward DW, Onwubiko HA, Priola SA, Caughey B. Ultrasensitive detection of scrapie prion protein using seeded conversion of recombinant prion protein. Nat Methods. 2007;4:645–650. - PubMed
-
- Bessen RA, Kocisko DA, Raymond GJ, Nandan S, Lansbury PT, Caughey B. Non-genetic propagation of strain-specific properties of scrapie prion protein. Nature. 1995;375:698–700. - PubMed
-
- Bocharova OV, Makarava N, Breydo L, Anderson M, Salnikov VV, Baskakov IV. Annealing prion protein amyloid fibrils at high temperature results in extension of a proteinase K-resistant core. J Biol Chem. 2006;281:2373–2379. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
