Optical imaging of contextual interactions in V1 of the behaving monkey
- PMID: 19587316
- PMCID: PMC2746790
- DOI: 10.1152/jn.90882.2008
Optical imaging of contextual interactions in V1 of the behaving monkey
Abstract
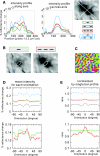
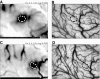
Interactions in primary visual cortex (V1) between simple visual elements such as short bar segments are believed to underlie our ability to easily integrate contours and segment surfaces. We used intrinsic signal optical imaging in alert fixating macaques to measure the strength and cortical distribution of V1 interactions among collinear bars. A single short bar stimulus produced a broad-peaked hill of activation (the optical point spread) covering multiple orientation hypercolumns in V1. Flanking the bar stimulus with a pair of identical collinear bars led to a strong nonlinear suppression in the optical signal. This nonlinearity was strongest over the center bar region, with a spatial distribution that cannot be explained by a simple gain control. It was a function of the relative orientation and separation of the bar stimuli in a manner tuned sharply for collinearity, being strongest for immediately adjacent bars lying on a smooth contour. These results suggest intracortical interactions playing a major role in determining V1 activation by smooth extended contours. Our finding that the interaction is primarily suppressive when imaged optically, which presumably reflects the combined inhibitory and excitatory inputs, suggests a complex interplay between these cortical inputs leading to the collinear facilitation seen in the spiking response of V1 neurons. This disjuncture between the facilitation seen in spiking and the suppression in imaging also suggests that cortical representations of complex stimuli involve interactions that need to be studied over extended networks and may be hard to deduce from the responses of individual neurons.
Figures








Similar articles
-
Nonlinear Lateral Interactions in V1 Population Responses Explained by a Contrast Gain Control Model.J Neurosci. 2018 Nov 21;38(47):10069-10079. doi: 10.1523/JNEUROSCI.0246-18.2018. Epub 2018 Oct 3. J Neurosci. 2018. PMID: 30282725 Free PMC article.
-
Input-output transformation in the visuo-oculomotor loop: comparison of real-time optical imaging recordings in V1 to ocular following responses upon center-surround stimulation.Arch Ital Biol. 2007 Nov;145(3-4):251-62. Arch Ital Biol. 2007. PMID: 18075119
-
Corticocortical feedback contributes to surround suppression in V1 of the alert primate.J Neurosci. 2013 May 8;33(19):8504-17. doi: 10.1523/JNEUROSCI.5124-12.2013. J Neurosci. 2013. PMID: 23658187 Free PMC article.
-
Scale-Invariant Visual Capabilities Explained by Topographic Representations of Luminance and Texture in Primate V1.Neuron. 2018 Dec 19;100(6):1504-1512.e4. doi: 10.1016/j.neuron.2018.10.020. Epub 2018 Nov 1. Neuron. 2018. PMID: 30392796 Free PMC article.
-
The dynamics of visual responses in the primary visual cortex.Prog Brain Res. 2007;165:21-32. doi: 10.1016/S0079-6123(06)65003-6. Prog Brain Res. 2007. PMID: 17925238 Review.
Cited by
-
Peripheral deafferentation-driven functional somatosensory map shifts are associated with local, not large-scale dendritic structural plasticity.J Neurosci. 2013 May 29;33(22):9474-87. doi: 10.1523/JNEUROSCI.1032-13.2013. J Neurosci. 2013. PMID: 23719814 Free PMC article.
-
Suppressive lateral interactions at parafoveal representations in primary visual cortex.J Neurosci. 2010 Sep 22;30(38):12745-58. doi: 10.1523/JNEUROSCI.6071-09.2010. J Neurosci. 2010. PMID: 20861379 Free PMC article.
-
Optimization and validation of a visual integration test for schizophrenia research.Schizophr Bull. 2012 Jan;38(1):125-34. doi: 10.1093/schbul/sbr141. Epub 2011 Oct 20. Schizophr Bull. 2012. PMID: 22021658 Free PMC article.
-
Decoding of coherent but not incoherent motion signals in early dorsal visual cortex.Neuroimage. 2011 May 15;56(2):688-98. doi: 10.1016/j.neuroimage.2010.04.011. Epub 2010 Apr 10. Neuroimage. 2011. PMID: 20385243 Free PMC article.
-
Imaging Cajal's neuronal avalanche: how wide-field optical imaging of the point-spread advanced the understanding of neocortical structure-function relationship.Neurophotonics. 2017 Jul;4(3):031217. doi: 10.1117/1.NPh.4.3.031217. Epub 2017 Jun 12. Neurophotonics. 2017. PMID: 28630879 Free PMC article. Review.
References
-
- Blakemore C, Tobin EA. Lateral inhibition between orientation detectors in the cat's visual cortex. Exp Brain Res 15: 439–440, 1972. - PubMed
-
- Bonhoeffer T, Grinvald A. Optical imaging based on intrinsic signals: the methodology. In: Brain Mapping: The Methods (2nd ed.), edited by Toga AW, Mazziotta JC. San Diego, CA: Academic Press, 1996, p. 97–140.
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

