Genetic dissection of rod and cone pathways in the dark-adapted mouse retina
- PMID: 19587322
- PMCID: PMC2746771
- DOI: 10.1152/jn.00142.2009
Genetic dissection of rod and cone pathways in the dark-adapted mouse retina
Abstract
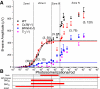
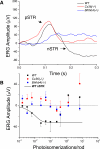
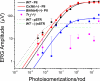
A monumental task of the mammalian retina is to encode an enormous range (>10(9)-fold) of light intensities experienced by the animal in natural environments. Retinal neurons carry out this task by dividing labor into many parallel rod and cone synaptic pathways. Here we study the operational plan of various rod- and cone-mediated pathways by analyzing electroretinograms (ERGs), primarily b-wave responses, in dark-adapted wildtype, connexin36 knockout, depolarizing rod-bipolar cell (DBCR) knockout, and rod transducin alpha-subunit knockout mice [WT, Cx36(-/-), Bhlhb4(-/-), and Tralpha(-/-)]. To provide additional insight into the cellular origins of various components of the ERG, we compared dark-adapted ERG responses with response dynamic ranges of individual retinal cells recorded with patch electrodes from dark-adapted mouse retinas published from other studies. Our results suggest that the connexin36-mediated rod-cone coupling is weak when light stimulation is weak and becomes stronger as light stimulation increases in strength and that rod signals may be transmitted to some DBCCs via direct chemical synapses. Moreover, our analysis indicates that DBCR responses contribute about 80% of the overall DBC response to scotopic light and that rod and cone signals contribute almost equally to the overall DBC responses when stimuli are strong enough to saturate the rod bipolar cell response. Furthermore, our study demonstrates that analysis of ERG b-wave of dark-adapted, pathway-specific mutants can be used as an in vivo tool for dissecting rod and cone synaptic pathways and for studying the functions of pathway-specific gene products in the retina.
Figures



References
-
- Applebury ML, Antoch MP, Baxter LC, Chun LL, Falk JD, Farhangfar F, Kage K, Krzystolik MG, Lyass LA, Robbins JT. The murine cone photoreceptor: a single cone type expresses both S and M opsins with retinal spatial patterning. Neuron 27: 513–523, 2000. - PubMed
-
- Bramblett DE, Pennesi ME, Wu SM, Tsai MJ. The transcription factor Bhlhb4 is required for rod bipolar cell maturation. Neuron 43: 779–793, 2004. - PubMed
-
- Calvert PD, Krasnoperova NV, Lyubarsky AL, Isayama T, Nicolo M, Kosaras B, Wong G, Gannon KS, Margolskee RF, Sidman RL, Pugh EN Jr, Makino CL, Lem J. Phototransduction in transgenic mice after targeted deletion of the rod transducin alpha-subunit. Proc Natl Acad Sci USA 97: 13913–13918, 2000. - PMC - PubMed
-
- Carter-Dawson LD, LaVail MM. Rods and cones in the mouse retina. I. Structural analysis using light and electron microscopy. J Comp Neurol 188: 245–262, 1979. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

