Hearing improvement after bevacizumab in patients with neurofibromatosis type 2
- PMID: 19587327
- PMCID: PMC4816642
- DOI: 10.1056/NEJMoa0902579
Hearing improvement after bevacizumab in patients with neurofibromatosis type 2
Abstract
Background: Profound hearing loss is a serious complication of neurofibromatosis type 2, a genetic condition associated with bilateral vestibular schwannomas, benign tumors that arise from the eighth cranial nerve. There is no medical treatment for such tumors.
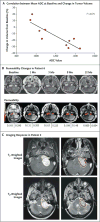
Methods: We determined the expression pattern of vascular endothelial growth factor (VEGF) and three of its receptors, VEGFR-2, neuropilin-1, and neuropilin-2, in paraffin-embedded samples from 21 vestibular schwannomas associated with neurofibromatosis type 2 and from 22 sporadic schwannomas. Ten consecutive patients with neurofibromatosis type 2 and progressive vestibular schwannomas who were not candidates for standard treatment were treated with bevacizumab, an anti-VEGF monoclonal antibody. An imaging response was defined as a decrease of at least 20% in tumor volume, as compared with baseline. A hearing response was defined as a significant increase in the word-recognition score, as compared with baseline.
Results: VEGF was expressed in 100% of vestibular schwannomas and VEGFR-2 in 32% of tumor vessels on immunohistochemical analysis. Before treatment, the median annual volumetric growth rate for 10 index tumors was 62%. After bevacizumab treatment in the 10 patients, tumors shrank in 9 patients, and 6 patients had an imaging response, which was maintained in 4 patients during 11 to 16 months of follow-up. The median best response to treatment was a volumetric reduction of 26%. Three patients were not eligible for a hearing response; of the remaining seven patients, four had a hearing response, two had stable hearing, and one had progressive hearing loss. There were 21 adverse events of grade 1 or 2.
Conclusions: VEGF blockade with bevacizumab improved hearing in some, but not all, patients with neurofibromatosis type 2 and was associated with a reduction in the volume of most growing vestibular schwannomas.
2009 Massachusetts Medical Society
Conflict of interest statement
No other potential conflict of interest relevant to this article was reported.
Figures

Comment in
-
Hearing improvement after bevacizumab for neurofibromatosis type 2.N Engl J Med. 2009 Oct 29;361(18):1809-10; author reply 1810-1. doi: 10.1056/NEJMc091694. N Engl J Med. 2009. PMID: 19864683 No abstract available.
-
Hearing improvement after bevacizumab for neurofibromatosis type 2.N Engl J Med. 2009 Oct 29;361(18):1810; author reply 1810-1. N Engl J Med. 2009. PMID: 19877310 No abstract available.
References
-
- Evans DG, Moran A, King A, Saeed S, Gurusinghe N, Ramsden R. Incidence of vestibular schwannoma and neurofibromatosis 2 in the North West of England over a 10-year period: higher incidence than previously thought. Otol Neurotol. 2005;26:93–7. - PubMed
-
- Samii M, Gerganov V, Samii A. Improved preservation of hearing and facial nerve function in vestibular schwannoma surgery via the retrosigmoid approach in a series of 200 patients. J Neurosurg. 2006;105:527–35. - PubMed
-
- Flickinger JC, Kondziolka D, Niranjan A, Lunsford LD. Results of acoustic neuroma radiosurgery: an analysis of 5 years’ experience using current methods. J Neurosurg. 2001;94:1–6. - PubMed
-
- Brackmann DE, Fayad JN, Slattery WH, III, et al. Early proactive management of vestibular schwannomas in neurofibromatosis type 2. Neurosurgery. 2001;49:274–80. - PubMed
-
- Combs SE, Volk S, Schulz-Ertner D, Huber PE, Thilmann C, Debus J. Management of acoustic neuromas with fractionated stereotactic radiotherapy (FSRT): long-term results in 106 patients treated in a single institution. Int J Radiat Oncol Biol Phys. 2005;63:75–81. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
