Effect of the monocyte chemoattractant protein-1/CC chemokine receptor 2 system on nephrin expression in streptozotocin-treated mice and human cultured podocytes
- PMID: 19587356
- PMCID: PMC2731530
- DOI: 10.2337/db08-0895
Effect of the monocyte chemoattractant protein-1/CC chemokine receptor 2 system on nephrin expression in streptozotocin-treated mice and human cultured podocytes
Abstract
Objective: Monocyte chemoattractant protein-1 (MCP-1), a chemokine binding to the CC chemokine receptor 2 (CCR2) and promoting monocyte infiltration, has been implicated in the pathogenesis of diabetic nephropathy. To assess the potential relevance of the MCP-1/CCR2 system in the pathogenesis of diabetic proteinuria, we studied in vitro if MCP-1 binding to the CCR2 receptor modulates nephrin expression in cultured podocytes. Moreover, we investigated in vivo if glomerular CCR2 expression is altered in kidney biopsies from patients with diabetic nephropathy and whether lack of MCP-1 affects proteinuria and expression of nephrin in experimental diabetes.
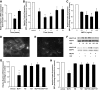
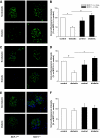
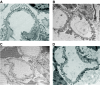
Research design and methods: Expression of nephrin was assessed in human podocytes exposed to rh-MCP-1 by immunofluorescence and real-time PCR. Glomerular CCR2 expression was studied in 10 kidney sections from patients with overt nephropathy and eight control subjects by immunohistochemistry. Both wild-type and MCP-1 knockout mice were made diabetic with streptozotocin. Ten weeks after the onset of diabetes, albuminuria and expression of nephrin, synaptopodin, and zonula occludens-1 were examined by immunofluorescence and immunoblotting.
Results: In human podocytes, MCP-1 binding to the CCR2 receptor induced a significant reduction in nephrin both mRNA and protein expression via a Rho-dependent mechanism. The MCP-1 receptor, CCR2, was overexpressed in the glomerular podocytes of patients with overt nephropathy. In experimental diabetes, MCP-1 was overexpressed within the glomeruli and the absence of MCP-1 reduced both albuminuria and downregulation of nephrin and synaptopodin.
Conclusions: These findings suggest that the MCP-1/CCR2 system may be relevant in the pathogenesis of proteinuria in diabetes.
Figures




References
-
- Molitch ME, DeFronzo RA, Franz MJ, Keane WF, Mogensen CE, Parving HH, Steffes MW: the American Diabetes Association. Nephropathy in diabetes. Diabetes Care 2004; 27: S79– S83 - PubMed
-
- Li JJ, Kwak SJ, Jung DS, Kim JJ, Yoo TH, Ryu DR, Han SH, Choi HY, Lee JE, Moon SJ, Kim DK, Han DS, Kang SW: Podocyte biology in diabetic nephropathy. Kidney Int 2007; 106: S36– S42 - PubMed
-
- Wolf G, Chen S, Ziyadeh FN: From the periphery of the glomerular capillary wall toward the center of disease: podocyte injury comes of age in diabetic nephropathy. Diabetes 2005; 54: 1626– 1634 - PubMed
-
- Pavenstadt H, Kriz W, Kretzler M: Cell biology of the glomerular podocyte. Physiol Rev 2003; 83: 253– 307 - PubMed
-
- Kestila M, Lenkkeri U, Mannikko M, Lamerdin J, McCready P, Putaala H, Ruotsalainen V, Morita T, Nissinen M, Herva R, Kashtan CE, Peltonen L, Holmberg C, Olsen A, Tryggvason K: Positionally cloned gene for a novel glomerular protein—nephrin—is mutated in congenital nephrotic syndrome. Mol Cell 1998; 1: 575– 582 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

