Laminopathies and the long strange trip from basic cell biology to therapy
- PMID: 19587457
- PMCID: PMC2701866
- DOI: 10.1172/JCI37679
Laminopathies and the long strange trip from basic cell biology to therapy
Abstract
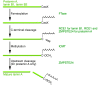
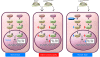
The main function of the nuclear lamina, an intermediate filament meshwork lying primarily beneath the inner nuclear membrane, is to provide structural scaffolding for the cell nucleus. However, the lamina also serves other functions, such as having a role in chromatin organization, connecting the nucleus to the cytoplasm, gene transcription, and mitosis. In somatic cells, the main protein constituents of the nuclear lamina are lamins A, C, B1, and B2. Interest in the nuclear lamins increased dramatically in recent years with the realization that mutations in LMNA, the gene encoding lamins A and C, cause a panoply of human diseases ("laminopathies"), including muscular dystrophy, cardiomyopathy, partial lipodystrophy, and progeroid syndromes. Here, we review the laminopathies and the long strange trip from basic cell biology to therapeutic approaches for these diseases.
Figures

References
Publication types
MeSH terms
Substances
Grants and funding
- R01 AR048997/AR/NIAMS NIH HHS/United States
- R01 NS059352/NS/NINDS NIH HHS/United States
- R01 CA099506/CA/NCI NIH HHS/United States
- R01 HL086683/HL/NHLBI NIH HHS/United States
- R01 AR050200/AR/NIAMS NIH HHS/United States
- NS059352/NS/NINDS NIH HHS/United States
- R01 AG025240/AG/NIA NIH HHS/United States
- CA099506/CA/NCI NIH HHS/United States
- AR048997/AR/NIAMS NIH HHS/United States
- HL76839/HL/NHLBI NIH HHS/United States
- AG025240/AG/NIA NIH HHS/United States
- R01 HL076839/HL/NHLBI NIH HHS/United States
- AR050200/AR/NIAMS NIH HHS/United States
- HL086683/HL/NHLBI NIH HHS/United States
- R56 NS059352/NS/NINDS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

