Engineering ligand-responsive gene-control elements: lessons learned from natural riboswitches
- PMID: 19587710
- PMCID: PMC5325117
- DOI: 10.1038/gt.2009.81
Engineering ligand-responsive gene-control elements: lessons learned from natural riboswitches
Abstract
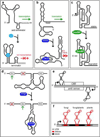
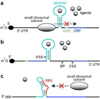
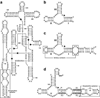
In the last two decades, remarkable advances have been made in the development of technologies used to engineer new aptamers and ribozymes. This has encouraged interest among researchers who seek to create new types of gene-control systems that can be made to respond specifically to small-molecule signals. Validation of the fact that RNA molecules can exhibit the characteristics needed to serve as precision genetic switches has come from the discovery of numerous classes of natural ligand-sensing RNAs called riboswitches. Although a great deal of progress has been made toward engineering useful designer riboswitches, considerable advances are needed before the performance characteristics of these RNAs match those of protein systems that have been co-opted to regulate gene expression. In this review, we will evaluate the potential for engineered RNAs to regulate gene expression and lay out possible paths to designer riboswitches based on currently available technologies. Furthermore, we will discuss some technical advances that would empower RNA engineers who seek to make routine the production of designer riboswitches that can function in eukaryotes.
Figures

References
-
- Gold L, Polisky B, Uhlenbeck O, Yarus M. Diversity of oligonucleotide functions. Annu Rev Biochem. 1995;64:763–797. - PubMed
-
- Osborne SE, Ellington AD. Nucleic acid selection and the challenge of combinatorial chemistry. Chem Rev. 1997;97:349–370. - PubMed
-
- Wilson DS, Szostak JW. In vitro selection of functional nucleic acids. Annu Rev Biochem. 1999;68:611–647. - PubMed
-
- Breaker RR. Engineered allosteric ribozymes as biosensor components. Curr Opin Biotechnol. 2002;13:31–39. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

