Synthesis at the molecular frontier
- PMID: 19587760
- PMCID: PMC2857510
- DOI: 10.1038/460197a
Synthesis at the molecular frontier
Abstract
Driven by remarkable advances in the understanding of structure and reaction mechanisms, organic synthesis will be increasingly directed to producing bioinspired and newly designed molecules.
Figures

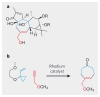

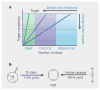
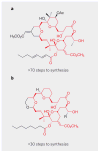
References
-
- Seebach D. Angew Chem Int Edn. 1990;29:1320–1367.
-
- Cornforth JW. Aust J Chem. 1993;46:157–170.
-
- Wender PA, Miller BL. In: Connectivity Analysis and Multibond-forming Processes in Organic Synthesis: Theory and Applications. Hudlicky T, editor. JAI Press; 1993. pp. 27–66.
-
- Tietze LF. Domino Reactions in Organic Synthesis. Wiley-VCH; 2006. - PubMed
-
- Nicolaou KC. Tetrahedron. 2003;59:6683–6738.
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

