Oocyte-specific deletion of Pten in mice reveals a stage-specific function of PTEN/PI3K signaling in oocytes in controlling follicular activation
- PMID: 19587782
- PMCID: PMC2702689
- DOI: 10.1371/journal.pone.0006186
Oocyte-specific deletion of Pten in mice reveals a stage-specific function of PTEN/PI3K signaling in oocytes in controlling follicular activation
Abstract
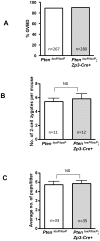
Immature ovarian primordial follicles are essential for maintenance of the reproductive lifespan of female mammals. Recently, it was found that overactivation of the phosphatidylinositol 3-kinase (PI3K) signaling in oocytes of primordial follicles by an oocyte-specific deletion of Pten (phosphatase and tensin homolog deleted on chromosome ten), the gene encoding PI3K negative regulator PTEN, results in premature activation of the entire pool of primordial follicles, indicating that activation of the PI3K pathway in oocytes is important for control of follicular activation. To investigate whether PI3K signaling in oocytes of primary and further developed follicles also plays a role at later stages in follicular development and ovulation, we conditionally deleted the Pten gene from oocytes of primary and further developed follicles by using transgenic mice expressing zona pellucida 3 (Zp3) promoter-mediated Cre recombinase. Our results show that Pten was efficiently deleted from oocytes of primary and further developed follicles, as indicated by the elevated phosphorylation of the major PI3K downstream component Akt. However, follicular development was not altered and oocyte maturation was also normal, which led to normal fertility with unaltered litter size in the mutant mice. Our data indicate that properly controlled PTEN/PI3K-Akt signaling in oocytes is essential for control of the development of primordial follicles whereas overactivation of PI3K signaling in oocytes does not appear to affect the development of growing follicles. This suggests that there is a stage-specific function of PTEN/PI3K signaling in mouse oocytes that controls follicular activation.
Conflict of interest statement
Figures



References
-
- McGee EA, Hsueh AJ. Initial and cyclic recruitment of ovarian follicles. Endocr Rev. 2000;21:200–214. - PubMed
-
- Broekmans FJ, Knauff EA, te Velde ER, Macklon NS, Fauser BC. Female reproductive ageing: current knowledge and future trends. Trends Endocrinol Metab. 2007;18:58–65. - PubMed
-
- Hansen KR, Knowlton NS, Thyer AC, Charleston JS, Soules MR, et al. A new model of reproductive aging: the decline in ovarian non-growing follicle number from birth to menopause. Hum Reprod. 2008;23:699–708. - PubMed
-
- Liu K, Rajareddy S, Liu L, Jagarlamudi K, Boman K, et al. Control of mammalian oocyte growth and early follicular development by the oocyte PI3 kinase pathway: new roles for an old timer. Dev Biol. 2006;299:1–11. - PubMed
-
- Cantley LC. The phosphoinositide 3-kinase pathway. Science. 2002;296:1655–1657. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

