Lack of IL-6 during coxsackievirus infection heightens the early immune response resulting in increased severity of chronic autoimmune myocarditis
- PMID: 19587788
- PMCID: PMC2703827
- DOI: 10.1371/journal.pone.0006207
Lack of IL-6 during coxsackievirus infection heightens the early immune response resulting in increased severity of chronic autoimmune myocarditis
Abstract
Background: Chronic myocarditis is often initiated by viral infection, the most common of which is coxsackievirus infection. The precise mechanism by which viral infection leads to chronic autoimmune pathology is poorly understood, however it is clear that the early immune response plays a critical role. Previous results have shown that the inflammatory cytokine interleukin (IL)-6 is integral to the development of experimental-induced autoimmune myocarditis. However, the function of IL-6 during viral-mediated autoimmunity has yet to be elucidated.
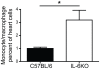
Methods and results: To address the requirement of IL-6 during disease induction, IL-6 deficient mice were infected with coxsackievirus B3 (CB3). Following infection, mice lacking IL-6 developed increased chronic autoimmune disease pathology compared to wild type controls without a corresponding change in the level of viral replication in the heart. This increase in disease severity was accompanied by elevated levels of TNF-alpha, MCP-1, IL-10, activated T cells and cardiac infiltrating macrophage/monocytes. Injection of recombinant IL-6 early following infection in the IL-6 deficient mice was sufficient to lower the serum cytokines TNF-alpha and IL-10 as well as the serum chemokines MCP-1, MIP-1beta, RANTES and MIG with a corresponding decrease in the chronic disease pathology strongly suggests an important regulatory role for IL-6 during the early response.
Conclusions: While IL-6 plays a pathogenic role in experimental-induced autoimmune disease, its function following viral-induced autoimmunity is not reprised. By regulating the early immune response and thereby controlling the severity of chronic disease, IL-6 directs the outcome of chronic autoimmune myocarditis.
Conflict of interest statement
Figures






Similar articles
-
Low-dose inorganic mercury increases severity and frequency of chronic coxsackievirus-induced autoimmune myocarditis in mice.Toxicol Sci. 2012 Jan;125(1):134-43. doi: 10.1093/toxsci/kfr264. Epub 2011 Oct 9. Toxicol Sci. 2012. PMID: 21984480 Free PMC article.
-
TLR3 deficiency induces chronic inflammatory cardiomyopathy in resistant mice following coxsackievirus B3 infection: role for IL-4.Am J Physiol Regul Integr Comp Physiol. 2013 Feb 15;304(4):R267-77. doi: 10.1152/ajpregu.00516.2011. Epub 2012 Dec 19. Am J Physiol Regul Integr Comp Physiol. 2013. PMID: 23255589 Free PMC article.
-
Mast cells and innate cytokines are associated with susceptibility to autoimmune heart disease following coxsackievirus B3 infection.Autoimmunity. 2004 Mar;37(2):131-45. doi: 10.1080/0891693042000196200. Autoimmunity. 2004. PMID: 15293883
-
Autoimmunity in coxsackievirus infection.Curr Top Microbiol Immunol. 2008;323:293-314. doi: 10.1007/978-3-540-75546-3_14. Curr Top Microbiol Immunol. 2008. PMID: 18357776 Review.
-
Molecular biology and pathogenesis of viral myocarditis.Annu Rev Pathol. 2008;3:127-55. doi: 10.1146/annurev.pathmechdis.3.121806.151534. Annu Rev Pathol. 2008. PMID: 18039131 Review.
Cited by
-
IL-6 promotes acute and chronic inflammatory disease in the absence of SOCS3.Immunol Cell Biol. 2012 Jan;90(1):124-9. doi: 10.1038/icb.2011.29. Epub 2011 Apr 26. Immunol Cell Biol. 2012. PMID: 21519345 Free PMC article.
-
The Antioxidant and Anti-Inflammatory Properties of Rice Bran Phenolic Extracts.Foods. 2020 Jun 24;9(6):829. doi: 10.3390/foods9060829. Foods. 2020. PMID: 32599964 Free PMC article.
-
Clinical presentation, pathogenesis, diagnosis, and treatment of epidermolysis bullosa acquisita.ISRN Dermatol. 2013 Jul 15;2013:812029. doi: 10.1155/2013/812029. eCollection 2013. ISRN Dermatol. 2013. PMID: 23956869 Free PMC article.
-
Biphasic and cardiomyocyte-specific IFIT activity protects cardiomyocytes from enteroviral infection.PLoS Pathog. 2019 Apr 8;15(4):e1007674. doi: 10.1371/journal.ppat.1007674. eCollection 2019 Apr. PLoS Pathog. 2019. PMID: 30958867 Free PMC article.
-
Autoimmune myocarditis, valvulitis, and cardiomyopathy.Curr Protoc Immunol. 2013;Chapter 15:Unit 15.14.1-51. doi: 10.1002/0471142735.im1514s101. Curr Protoc Immunol. 2013. PMID: 23564686 Free PMC article.
References
-
- Eriksson U, Kurrer MO, Schmitz N, Marsch SC, Fontana A, et al. Interleukin-6-deficient mice resist development of autoimmune myocarditis associated with impaired upregulation of complement C3. Circulation. 2003;107:320–325. - PubMed
-
- Kanda T, McManus JE, Nagai R, Imai S, Suzuki T, et al. Modification of viral myocarditis in mice by interleukin-6. Circ Res. 1996;78:848–856. - PubMed
-
- Kanda T, Takahashi T. Interleukin-6 and cardiovascular diseases. Jpn Heart J. 2004;45:183–193. - PubMed
-
- Tanaka T, Kanda T, McManus BM, Kanai H, Akiyama H, et al. Overexpression of interleukin-6 aggravates viral myocarditis: impaired increase in tumor necrosis factor-alpha. J Mol Cell Cardiol. 2001;33:1627–1635. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

