Staphylococcal enterotoxin A induces small clusters of HLA-DR1 on B cells
- PMID: 19587800
- PMCID: PMC2705189
- DOI: 10.1371/journal.pone.0006188
Staphylococcal enterotoxin A induces small clusters of HLA-DR1 on B cells
Abstract
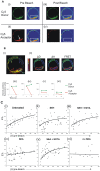
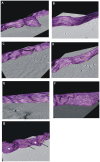
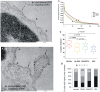
The superantigen SEA causes non-specific hyperactivation of T and B cells at low concentrations. Studies of mutants or soluble proteins suggest SEA is bivalent for its ligand, MHC class II. However, the interaction between these molecules on intact cells is unknown. On primary mouse B cells expressing the MHC class II allele HLA-DR1, measurements of Förster Resonance Energy Transfer between HLA-DR1 molecules on SEA-treated cells indicated specific clustering, not observed in untreated or monovalent superantigen treated cells. Tomographic visualization and electron microscopy of immunogold-labeled SEA-treated B cells revealed small clusters of surface HLA-DR1 (< or = 4 gold labels). These results present direct visual evidence of SEA-mediated clustering of MHC class II molecules on treated antigen presenting cells, and provide a new structural approach to addressing problems of this nature.
Conflict of interest statement
Figures
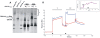
References
-
- Fraser JD. High-affinity binding of staphylococcal enterotoxins A and B to HLA-DR. Nature. 1989;339:221–223. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

