Cutaneous chronic graft-versus-host disease does not have the abnormal endothelial phenotype or vascular rarefaction characteristic of systemic sclerosis
- PMID: 19587802
- PMCID: PMC2705674
- DOI: 10.1371/journal.pone.0006203
Cutaneous chronic graft-versus-host disease does not have the abnormal endothelial phenotype or vascular rarefaction characteristic of systemic sclerosis
Abstract
Background: The clinical and histologic appearance of fibrosis in cutaneous lesions in chronic graft-versus -host disease (c-GVHD) resembles the appearance of fibrosis in scleroderma (SSc). Recent studies identified distinctive structural changes in the superficial dermal microvasculature and matrix of SSc skin. We compared the dermal microvasculature in human c-GVHD to SSc to determine if c-GVHD is a suitable model for SSc.
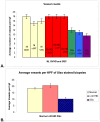
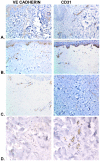
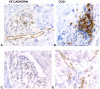
Methodology/principal findings: We analyzed skin biopsies of normal controls (n = 24), patients with SSc (n = 30) and c-GVHD with dermal fibrosis (n = 133)). Immunostaining was employed to identify vessels, vascular smooth muscle, dermal matrix, and cell proliferation. C-GVHD and SSc had similar dermal matrix composition and vascular smooth muscle pathology, including intimal hyperplasia. SSc, however, differed significantly from c-GVHD in three ways. First, there were significantly fewer (p = 0.00001) average vessels in SSc biopsies (9.8) when compared with c-GVHD (16.5). Second, in SSc, endothelial markers were decreased significantly (19/19 and 12/14 for VE cadherin and vWF (p = <0.0001 and <0.05), respectively). In contrast, 0/13 c-GVHD biopsies showed loss of staining with canonical endothelial markers. Third, c-GVHD contained areas of microvascular endothelial proliferation not present in the SSc biopsies.
Conclusions/significance: The sclerosis associated with c-GVHD appears to resemble wound healing. Focal capillary proliferation occurs in early c-GVHD. In contrast, loss of canonical endothelial markers and dermal capillaries is seen in SSc, but not in c-GVHD. The loss of VE cadherin in SSc, in particular, may be related to microvascular rarefaction because VE cadherin is necessary for angiogenesis. C-GVHD is a suitable model for studying dermal fibrosis but may not be applicable for studying the microvascular alterations characteristic of SSc.
Conflict of interest statement
Figures


References
-
- Filipovich AH, Weisdorf D, Pavletic S, Socie G, Wingard JR, Lee S, et al. National Institutes of Health consensus development project on criteria for clinical trials in chronic graft-versus-host disease: I. Diagnosis and staging working group report. Biol Blood Marrow Transplant. 2005;11:945–956. - PubMed
-
- Hymes SR, Turner M, Champlin R, Couriel D. Cutaneous manifestations of chronic graft-versus-host disease. Biol Blood Marrow Transplant. 2006;12:1101–1113. - PubMed
-
- Shulman HM, Kleiner D, Lee SJ, Morton T, Pavletic SZ, Farmer E, et al. Histopathologic diagnosis of chronic graft-versus-host disease: National Institutes of Health Consensus Development Project on Criteria for Clinical Trials in Chronic Graft-versus-Host Disease: II. Pathology Working Group Report. Biol Blood Marrow Transplant. 2006;12:31–47. - PubMed
-
- Shlomchik WD. Graft-versus-host Disease. Nat Rev Immunol. 2007;7:340–352. - PubMed
-
- Zhang Y, McCormick LL, Desai SR, Wu C, Gilliam AC. Murine sclerodermatous graft-versus-host disease, a model for human scleroderma: cutaneous cytokines, chemokines, and immune cell activation. J Immunol. 2002;168:3088–3098. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

